Fig. 48.1
Air enema showing intussusception up to the rectum
Spontaneous reduction is another possible outcome and reportedly occurs in almost 20 % of intussusceptions [4].
Etiology
The pathogenesis of intussusception is possibly caused by an imbalance in the longitudinal forces acting along the intestinal wall.
The majority of intussusceptions (~ 95 %) are idiopathic as no obvious etiology can be identified. In these so-called idiopathic cases, it is thought that Peyer’s patches, hypertrophied in response to a respiratory or gastrointestinal infection, function as a lead point . Peyer’s patches are oval masses of aggregated lymphoid follicles on the mucous membrane lining the small intestine. Peyer’s patches are distributed irregularly along the anti-mesenteric wall, becoming more numerous and forming a lymphoid ring in the distal ileum. These structures have been labeled the “immune sensors of the intestine’ owing to their role in “sampling” the contents of the gut lumen, taking up antigens and microorganisms and, if appropriate, stimulating a protective mucosal immune response [5]. This probably explains why an antecedent viral infection is present in as many as 20 % of intussusceptions. Specifically, adenovirus [6], cytomegalovirus, and live rotavirus vaccines [7] have been variably associated with intussusception. Bacterial enteritis involving, for example, Salmonella, E. coli, Shigella, and Campylobacter, also increases the risk of intussusception in children [8].
This imbalance may be caused by a mass protruding into the intestinal lumen, which represents a “lead point” upon which peristalsis acts in an attempt to clear it as if it were a bolus of food. A pathological lead point has been defined as “a recognizable intraperitoneal anomaly or abnormality that tethers or obstructs the bowel, initiating the process of intussusception” [4]. Pathological lead points are identified in 2–12 % of intussusceptions. While a lead point is rarely identified in patients < 2 years, 20 % of patients > 2 years are found to have a lead point [2]. Pathological lead points are more commonly identified in ileoileal or colocolic intussusceptions.
Meckel’s diverticulum [9], benign and malignant intestinal or mesenteric tumors including lipomas [10], lymphomas [11], and polyps associated with Peutz–Jeghers syndrome [12], duplication cysts [13, 14], intestinal abnormalities associated with cystic fibrosis (e.g., hypertrophied mucosal glands or thickened feces) [15], hematomas secondary to abdominal trauma [16] or—Henoch–Schönlein purpura and other vascular/coagulation disorders [17], foreign bodies [18], intestinal hemangiomas [19], Kaposi sarcoma [20], abnormalities associated with posttransplantation lymphoproliferative disorder [21], and anastomotic sutures and staples and indwelling tubes [22] have all been reported as providing pathological lead points.
A small percentage of intussusceptions (typically ileoileal) arise postoperatively, usually after laparotomy with extensive bowel manipulation, although it has been reported following other abdominal and non-abdominal procedures. The precise mechanism underlying postoperative intussusception is unknown, but disorganized peristalsis, early postoperative adhesions, electrolyte disturbances, anesthetic drugs, and/or neurogenic factors may be implicated [23].
Epidemiology
In the UK, the reported incidence of intussusception stands at around 1.6–4 cases per 1000 live births [2]. Males are affected more frequently than females, with an incidence ratio of 3:2. The male preponderance becomes more evident after 9 months of age.
Ninety percent of intussusceptions will occur within the first 3 years of life, 65 % of cases arising within the first year of life and 50 % between 3 and 10 months [2]. Incidence peaks between 5 and 7 months [24]. Intussusception in utero is rarely reported [25, 26], and perinatal intussusception in newborns accounts for only 0.3 % of all cases. Several reasons for the increase in incidence from around 3 months of age have been advanced, including changes in feeding practices that affect the gut, maturation of lymphoid tissue, fattening of the mesentery which increases the likelihood of it becoming trapped, or a decline in the protection afforded by maternal antibodies against microorganisms that might precipitate intussusception [2, 27] .
Adult intussusception is rare, representing around 5 % of all cases. Unlike in the pediatric population, where intussusception is the most common cause of acute intestinal obstruction and is usually idiopathic, intussusception only accounts for 1–5 % of cases of intestinal obstruction in adults, often presents atypically and subacutely [28], and it is attributable to a pathological process in 90 % of cases. A more definitive, surgical approach (often resection) is warranted when managing adult intussusception compared to pediatric intussusception, due to the significant risk of associated malignancy (~ 65 % cases) [29] and high risk of perforation and leakage of microorganisms [30].
Evidence suggests that the relative risk of intussusception may vary according to race or ethnicity. For example, in their review of pediatric hospitalization data in the USA between 1993 and 2004, Tate et al. [31] found that in infants over 16 weeks of age, non-Hispanic black and Hispanic infants had higher rates of hospitalization for intussusception compared with non-Hispanic white infants. In line with the USA findings, research from the UK and Republic of Ireland suggests that Black Caribbean and African infants have higher incidence rates of intussusception than in White British and Asian groups [27]. Justice et al. [32] and Webby et al. [33] have identified a lower risk of intussusception among indigenous Australian children compared to nonindigenous children. However, as these studies rely on data gathered from hospitals, the differences they identify may reflect differences in admission and access rather than any actual ethnic variation in intussusception incidence [34].
Incidence of intussusception is also thought to vary by geographic region. Compared to other regions, it has been observed that incidence is higher than average in populations in Australia, Hong Kong, Vietnam, South Korea, and Japan and lower than average in populations in Finland, India, Malaysia, and Bangladesh [24, 35]. However, data upon which the assertion of geographic variability is based is problematic [27].
Finally, it is suggested that incidence of intussusception varies by season. Incidence has been found to peak during winter (Dec–Feb) and spring (March–May) in the UK and Republic of Ireland [27], although no such trend has been demonstrated in studies in equatorial regions [36] or indeed in those performed on a global scale [24]. Absence of any significant seasonal variation in incidence of intussusception goes against there being any strong association between intussusception and natural rotavirus, which has a highly seasonal pattern [37] .
Classification
Intussusception is classified anatomically, with the proximal portion of the intestine (the “intussusceptum”) first, followed by the more distal portion (the “intussuscepiens”). The majority of intussusceptions (~80 %) involve the terminal ileum telescoping into the cecum or ascending colon and are thus termed ileocecal or ileocolic intussusceptions. Less frequently, segments of ileum invaginate into ileum (ileoileal) or segments of colon invaginate into colon (colocolic) or a combination arises (ileo-ileo-colic).
Clinical Presentation
Sudden onset of severe, colicky abdominal pain is the most common presenting feature of intussusception [38], present in around 85 % of cases [39]. Infants will typically present with episodes of inconsolable crying while drawing up their legs in conjunction with spasms of peristalsis. These episodes occur every 10–15 min and last around 2–3 min. The pain becomes more constant after around 12 h. Between episodes, the infant may appear normal or increasingly pale, clammy, quiet, and lethargic. It is hypothesized that lethargy may be induced by the release of endogenous opioids or endotoxins from the ischemic bowel [40].
Vomiting (non-bilious, undigested gastric contents becoming bilious) can be an early indication of intestinal obstruction. Evacuation of small, loose stools from the colon distal to the obstruction will occur early in the course of the disease in some patients. Around 50 % of patients pass “red currant jelly” stool [39]. Overall, 1/3 patients will have the classic triad of abdominal pain, vomiting, and bloody stool [2].
The combination of reduced fluid intake, increased fluid loss through vomiting, anticipated losses into the obstructed bowel, and perhaps some reactive vasodilation can culminate in dehydration and hypovolemic shock. If the bowel perforates resulting in bacteremia, the child will become febrile, tachycardic, and hypotensive.
Examination can be unremarkable in between “attacks.” A “sausage-shaped” mass is palpable in around 65 % of cases, usually in the right upper quadrant extending to the left along the line of the transverse colon (Fig. 48.2). The mass can be tender and is sometimes seen on clinical inspection. It can become harder to detect this mass as the disease progresses and the abdomen distends [39]. The right lower quadrant can become flat or empty due to the absence of bowel (“Dance” sign). In around 5 % of cases, the apex of the intussusceptum can be palpated on rectal examination. It rarely prolapses out of the anus.
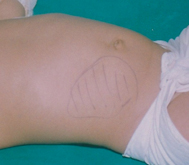

Fig. 48.2
Typical right abdominal mass
Differential Diagnosis
Conditions that mimic features of intussusception are shown below. These differential diagnoses should be excluded on the basis of careful history taking and examination (Table 48.1).
Differential diagnosis | Supporting | Excluding |
|---|---|---|
Infantile colic due to wind in the intestine associated with feeding difficulties | Common in first 3 months | Rarely lasts 1 h Usually no vomiting |
Gastroenteritis | Severe cases can present with colic and passage of blood and mucus | Greater volume of diarrhea |
Strangulated inguinal hernia | Abdominal pain and distension Vomiting | On examination – irreducible lump in the groin |
Investigation
Diagnostic work up of a patient with clinically suspected intussusception might include a plain abdominal radiograph, abdominal ultrasound, contrast enema, and CT scan. Laboratory tests are not specifically diagnostic but may show leukocytosis, acidosis, and electrolyte disturbance associated with bowel ischemia.
A plain radiograph is usually only performed if the diagnosis on presentation is unclear [3]. Suggestive signs of intussusception on a plain radiograph include an elongated soft tissue mass typically in the right upper quadrant, abnormal distribution of gas and fecal contents, dilated bowel loops, no gas in the transverse or descending colon, and air–fluid levels in the presence of bowel obstruction. Although plain radiographs can aid diagnosis of intussusception, they may appear normal in the early stages and they lack sensitivity (i.e., high incidence of false positives) [41].
Ultrasonography is an extremely effective imaging modality for diagnosing intussusception. It has a sensitivity over 98 % and a specificity of 100 % when diagnosing ileocolic or colocolic intussusceptions. These parameters are slightly reduced for ileoileal intussusceptions [2]. The “doughnut” or “target” sign on transverse section (concentric rings created by the telescoping bowel) and a “pseudo-kidney” (bowel wall and mesentery mimic renal structures) are characteristic signs of intussusception on ultrasound [2].
If the diagnosis is still in doubt, a contrast enema is the gold standard for diagnosing intussusception. Barium or (more commonly) air is introduced via a catheter inserted into the rectum. When the contrast substance enters the lumen of the intussusceptum and the intraluminal space, this creates the “coiled-spring” sign. This test is contraindicated if there is evidence of perforation on plain radiograph. Enemas can be therapeutic as well as diagnostic [41].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







