Fig. 12.1
Transducer probes – (a) Handheld side-fire curvilinear-array transducer. (b) Handheld end-fire linear-array transducer. (c) Laparoscopic side-fire linear-array transducer. (d) Hand-assisted laparoscopic approach
Laparoscopic transducers are either fixed or articulating (generally with 6° of freedom) side-fire linear or curvilinear arrays operating at a slightly lower frequency range of 5–10 MHz. The use of a laparoscopic transducer with an articulating head increases the ability of the operator to view different anatomic regions of the pancreas through the same port (Fig. 12.1c).
A hand-assisted laparoscopic approach should be considered if accurate laparoscopic imaging is difficult to obtain. This hybrid technique allows the use of handheld side-fire transducers to view anatomy often impossible to view with traditional laparoscopic access while still maintaining many of the benefits of laparoscopic resection. However, this added variability should not preclude well-thought-out preoperative patient, equipment, and port placement (Fig. 12.1d).
Preoperative Setup
Proper setup can significantly reduce case length and operator stress and improve patient outcomes. The patient should be supine on an operating table in a neutral position. The ultrasound monitor should be placed in a direct line-of-sight across from the operator (Fig. 12.2). If laparoscopic instruments are to be utilized, their monitors should be placed directly next to or above the ultrasound monitor. Modern laparoscopic and ultrasound equipment provide a “picture-in-picture” feature that allows viewing of ultrasonic images within a dedicated space on the laparoscopic monitor (see Fig. 12.3). The monitor should be at eye level and in the line-of-sight to reduce operator neck and/or eyestrain. When using a fixed laparoscopic probe, port placement should be well planned before the patient is prepped. Table 12.1 lists the common port placement locations and the associated anatomic region best visualized in this location when a fixed probe is utilized. The use of a laparoscopic probe with an articulating head can usually scan the pancreas in two planes when placed anywhere in the abdomen.
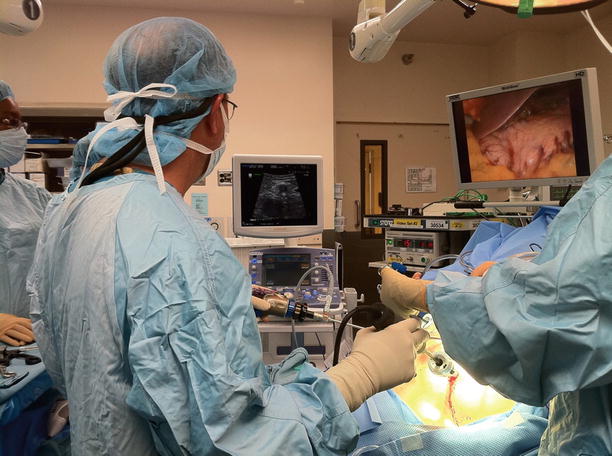
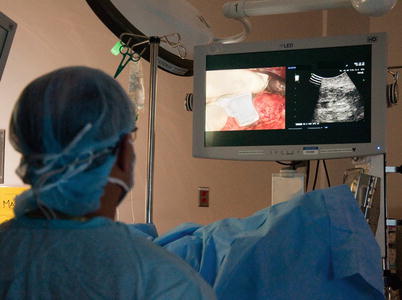

Fig. 12.2
Line-of-sight viewing – Aligning laparoscopic and ultrasound monitors in an ergonomically advantageous position will reduce operator stress

Fig. 12.3
Picture-in-picture – Modern laparoscopic equipment may have picture-in-picture capabilities, allowing for line-of-sight viewing without an additional monitor
Table 12.1
Ports placed in the locations listed below provide optimal viewing of the corresponding anatomic regions of the pancreas when using a fixed laparoscopic transducer
Port location | View of the pancreas best provided |
|---|---|
Umbilicus | Longitudinal images of the portal vein and common bile duct |
Transverse images of the pancreas neck, body | |
Right upper quadrant | Transverse images of the pancreatic tail |
Longitudinal, axial images of the pancreatic head, neck, tail | |
Left upper quadrant | Oblique images of the pancreatic head |
Scanning Techniques
The timing and method of pancreatic intraoperative ultrasonographic evaluation should be carefully planned. If the operative goal is disease staging, then IOUS should be performed immediately after entering the abdomen to assess for metastasis and local invasion that would prohibit resection. In patients with limited intra-abdominal fat, ultrasonographic views of the pancreas may be obtained via indirect acoustic coupling through the stomach, duodenum, mesocolon, or liver by utilizing low frequency and steady compression of overlying structures (Fig. 12.4). The use of acoustic coupling allows imaging of pertinent structures without disrupting anatomic planes. For cases in which patient anatomy precludes indirect viewing or violation of anatomic spaces is not a concern, direct imaging of the exposed pancreas is preferred for superior resolution (Fig. 12.5). Since a direct scan does not need to penetrate through overlying structures, a higher frequency may be utilized. It is important that minimal compression of the pancreas be performed with all scanning techniques, as even light compression can limit the ability to accurately view surface lesions and pancreatic ductal anatomy in a soft gland. Imaging of surface lesions may be improved by utilizing a “probe-standoff” technique, in which the field to be viewed is flooded with sterile saline and the transducer is immersed within this conductive medium and held just off the area of interest. Alternatively, a fluid-filled glove can be placed between the transducer and the gland to provide the conduction medium. Both techniques facilitate excellent acoustic coupling without the need to compress the gland (Fig. 12.6).
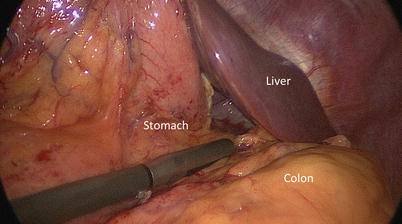
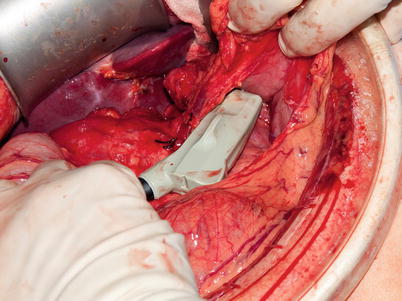
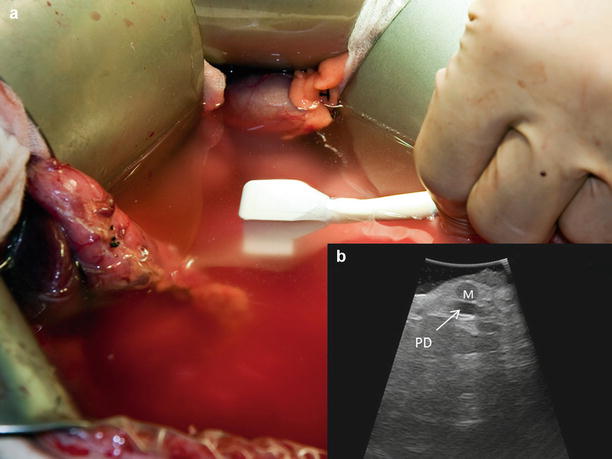

Fig. 12.4
Indirect scanning – The pancreas may be viewed through surrounding structures via acoustic coupling. This allows for initial evaluation of pathology with minimal disruption of anatomic planes

Fig. 12.5
Direct scanning – Superior imaging of the pancreas and surrounding structures is obtained via placement of the probe directly on the organ’s surface

Fig. 12.6
Standoff technique – By immersing the probe in saline (a), a view of the surface of the gland is obtained without compression. This method (b) offers superior imaging of a surface lesion (M) and small pancreatic duct (PD)
Once the choice between indirect and direct visualization has been made, the next focus of examination should be complete assessment of anatomic structures. This is best achieved via systematic scanning of the organ in both and transverse planes. The longitudinal plane is also referred to as “sagittal” and is obtained with the probe oriented along the long axis of the pancreas. Similarly, the transverse plane is also known as “axial” and is obtained with the probe oriented along the short axis (Fig. 12.7). Overlapping sweeps of the gland in both planes should begin at the head and work toward the tail on the ventral surface, providing longitudinal and cross-sectional views of the main pancreatic duct and parenchyma. Examination of the head and/or uncinate process may benefit from additional scanning from the right lateral or anterolateral aspect. Visualization of the intrapancreatic and/or periampullary bile duct is best achieved via acoustic coupling transduodenally (Fig. 12.8). The duodenal luminal gas is usually easily compressed with the probe to provide adequate imaging. Rarely, a nasogastric tube may be required to introduce saline into the duodenum to displace the luminal gas or a Kocher maneuver employed to provide a more lateral approach to the periampullary region. Lateral movement, rotation, angulation, and swing maneuvers (see Table 12.2 for definitions) may be employed to visualize key structures listed in the normal anatomy section below. Color Doppler may be employed if evaluation of vessel patency is of clinical importance. (Video 12.1 depicts laparoscopic pancreas scanning technique.)
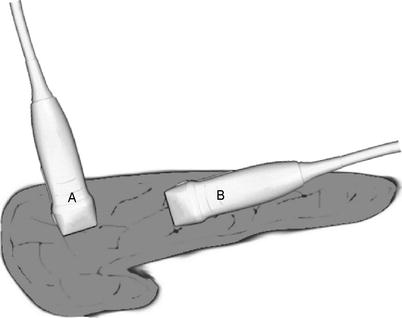
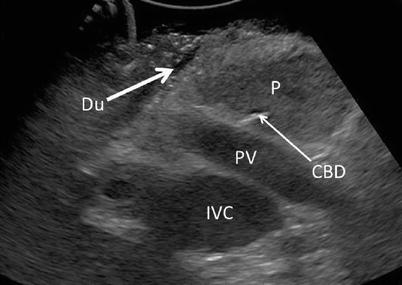

Fig. 12.7
Probe orientation – The transducer may be used to provide images in either a longitudinal (sagittal) plane (A) or in a transverse (axial) plane (B)

Fig. 12.8
Transduodenal view – By placing the probe anterolaterally on the duodenum (Du), an excellent view of the pancreatic head (P) at the level of the portal vein (PV) may be obtained with compression. Common bile duct (CBD), inferior vena cava (IVC), and portal vein (PV)
Table 12.2
The various movements utilized in the systematic scanning of pancreatic structures
Maneuver | Description of technique |
|---|---|
Lateral movement | Lateral movement of the probe along either the transverse or longitudinal path of the structure, with the probe in constant contact with the structure’s surface. The most common technique during scanning |
Rotation | Rotation of the probe along the direction of the ultrasonic beam. May be utilized to change between transverse and longitudinal views without having to pick up the probe |
Angulation | The transducer surface is kept fixed on the organ, while the angle of the ultrasound beam is changed by pivoting the probe along its long axis. Utilized to obtain three-dimensional information or within confined spaces |
Swing | Using the probe cable as a fulcrum, the probe head is swung in a pendulous motion while in contact with the structure surface. May be utilized in either transverse or longitudinal pathways |
Normal Pancreatic Anatomy
Normal pancreatic parenchyma should have a homogeneous echogenicity similar to the liver, and the pancreatic duct should appear hypoechoic with well-defined borders (Fig. 12.9). The confluence of the splenic vein and superior mesenteric vein should be well visualized as it transitions to the portal vein beneath the neck. The relationship between the pancreatic duct, common bile duct, and gastroduodenal artery should be delineated. The aorta, inferior vena cava, celiac plexus, and superior mesenteric artery should all be visible as the surface of the pancreas is scanned. Doppler imaging may be useful in confirming structures (Fig. 12.10).
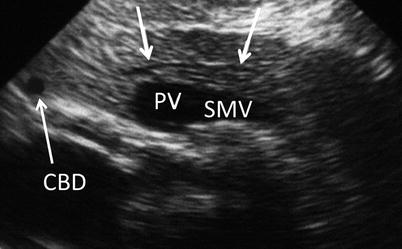
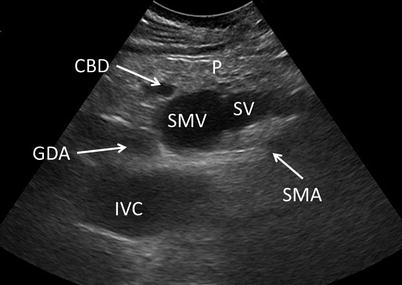

Fig. 12.9
Normal ductal anatomy – A normal main pancreatic duct (white arrows) is visualized in a longitudinal view. Also seen are the common bile duct (CBD) and the confluence of the portal (PV) and superior mesenteric veins (SMV) (With kind permission from Lichtenstein [76])

Fig. 12.10
Normal vessel anatomy – Vasculature visible through the head and neck of the pancreas (P) should include: the superior mesenteric artery (SMA) and vein (SMV), inferior vena cava (IVC), splenic vein (SV), and gastroduodenal artery (GDA). The aorta, portal vein, and splenic artery may also be visible in alternate planes. The common bile duct (CBD) is seen in this image
Benign fatty infiltration of the pancreas is becoming more common with increasing Body Mass Indexes and appears as diffuse hyperechoic appearance of the gland often with head or uncinate sparing (Fig. 12.11). This sparing anomaly is thought to be due to the different embryologic origins of the dorsal and ventral pancreatic buds. It is important to understand this differentiation as this contrast in relative echogenicities can be misinterpreted as a mass [8].
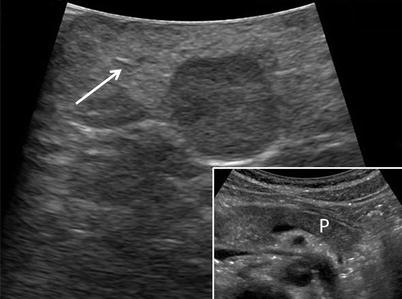

Fig. 12.11
Fatty infiltrate – Fatty infiltration of the pancreas showing diffuse hyperechogenicity of pancreatic parenchyma (white arrow) compared to normal parenchyma (P) shown within inset
Guidance Techniques
One of the key benefits of IOUS over other imaging modalities is its ability to provide real-time imaging guidance for needle localization or tissue dissection. Needle localization is often employed to locate the pancreatic duct prior to exposure or to aspirate cystic structures for analysis. Specialty devices are available commercially to aid in needle placement; however, a significantly cheaper freehand approach is similarly effective. With the freehand method the structure of interest is first identified with the ultrasound transducer, its center aligned with center of the probe, and the approximate anatomic depth noted. This can be done in either longitudinal or transverse planes, but the former will allow visualization of needle advancement through the entire gland. A long 21- to 27-gauge needle is then placed at an equidistance from the structure of interest related to the depth, in the plane between the operator and the probe, and aligned with the center of the probe (Fig. 12.12). The needle is then advanced under ultrasound guidance at an approximate 45° angle into the structure of interest. A syringe may be attached to the finder needle at this point and gently aspirated to confirm placement into a duct or cyst if relevant. If the intent is to expose the pancreatic duct, the needle may then be utilized as a guide for cut-down with electrocautery if the course of the duct is evident. If the duct is narrow and difficult to visualize, an appropriately sized wire may be advanced through the needle in order to cannulate the entire length of the duct and subsequently utilized for exposure.
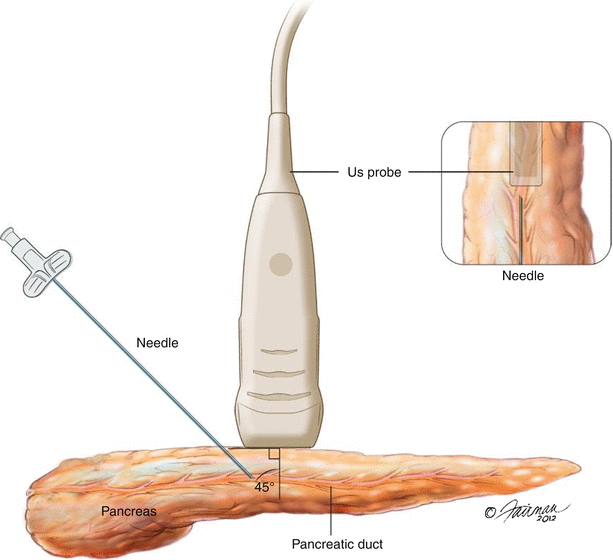

Fig. 12.12
Needle guidance – The needle should be placed in-line with the probe, enter the gland at a distance equal to the depth of the lesion, and follow a 45° angle. The needle tip should be visible throughout the advancement
Contrast Enhancement
The use of contrast-enhanced ultrasound (CEUS) is relatively new to the surgeon’s armamentarium, with the first reports of its clinical use published in 2000 [9]. The initial application of this technology was limited to the evaluation of the right heart due to the first generation of contrast agents being destroyed after passing through the pulmonary circulation. Second-generation contrast agents are more stable, can be administered peripherally, and have indications in evaluating a variety of organ systems [10]. While the main utility of this technique has been in the investigation of liver lesions, it has found some use in differentiating pancreatic lesions. The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) periodically releases guidelines for CEUS, with its last revision in 2011 [11]. Based on the recommendations of the EFSUMB, CEUS has a sufficient level of evidence for use in the following pancreatic conditions:
1.
Characterizing ductal adenocarcinomas (evidence level: A;1b)
2.
Differentiating pseudocysts from cystic tumors (evidence level: A;1b)
3.
Differentiating solid from liquid/necrotic components of a lesion (evidence level: A;1b)
4.
Defining lesion dimensions and anatomic relationships with surrounding structures (evidence level: B;2b)
Contrast enhancement of the pancreatic arteries begins immediately after aortic enhancement, lasts 10–30 s, and is immediately followed by a 90-s venous phase [12]. The liver should then be assessed for metastasis after the pancreatic venous phase, using the same contrast injection [13]. The specific ultrasonic findings for each indication will be discussed below in the corresponding pathologic section. Although there is no significant evidence to recommend the routine use of CEUS to evaluate pancreatic lesions, the technique should be considered if previous diagnostic work-up is equivocal. (See Chap. 23, section “Contrast-enhanced ultrasound,” for more information.)
Condition-Specific Indications
The following sections will discuss indications of IOUS for various pancreatic pathologies and focus on their typical ultrasonographic features. Images were obtained via handheld and/or LUS.
Pancreatitis
Indications: Operative treatment of acute and/or chronic pancreatitis and its major sequelae have been on the decline with the advancement of various percutaneous and endoscopic treatments such as dual drainage and rendezvous techniques [14, 15]. However, for lesions not amenable to these techniques or for institutions without access to advanced subspecialist, operative drainage of pseudocysts or abscesses, debridement of necrotic gland, or treatment of pseudoaneurysm may be required. Ultrasonographic localization of the main pancreatic duct (see section “Guidance techniques”) should be considered during any Puestow or Frey procedure in which the pancreatic duct is not easily palpable.
Acute Pancreatitis Findings: Generally shows hypoechogenicity or a mixed echo pattern of the parenchyma due to edema or associated necrotic and hemorrhagic tissue. CEUS may be utilized to delineate non-enhancing areas of necrosis for debridement [16].
Chronic Pancreatitis Findings: Non-autoimmune etiologies are characterized by heterogeneous hyperechogenicity of a hard and atrophic parenchyma, frequently associated with calcifications and acoustic shadowing. The pancreatic duct appears hypoechoic, is often dilated (can appear as a series of dilations and strictures, the so-called chain of lakes), and may contain intraductal calcifications with associated acoustic shadowing (Fig. 12.13). This is in stark contrast to autoimmune pancreatitis which is characterized by heterogenic hypoechogenicity of an enlarged gland, often with a strictured duct, and rare calcifications (Fig. 12.14).
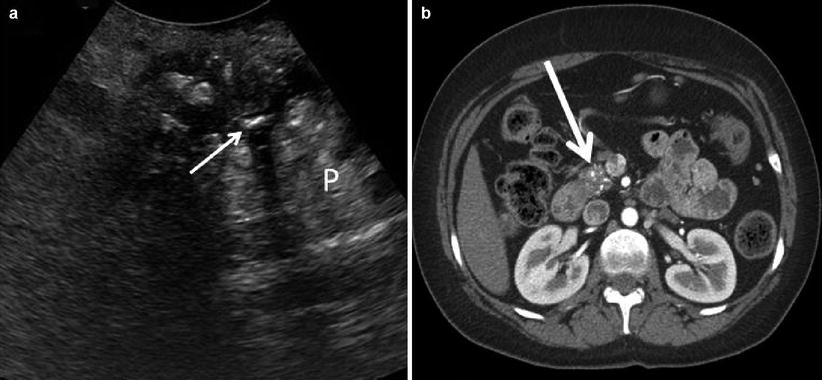
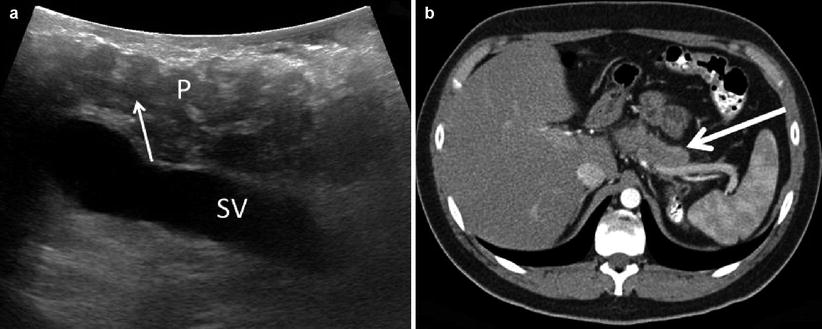

Fig. 12.13
Chronic pancreatitis – A transverse view (a) shows a hyperechoic parenchyma with calcifications (P) and a narrow pancreatic duct stone with acoustic shadowing (thin white arrow). A corresponding CT scan (b) shows an atrophic head with multiple calcifications (thick white arrow)

Fig. 12.14
Autoimmune pancreatitis – A longitudinal view (a) shows a hypoechoic parenchyma (P) and a narrow pancreatic duct (thin white arrow). The splenic (SV) vein is also noted. A corresponding CT scan (b) shows a thickened gland with a smooth surface (thick white arrow), often described as “sausage-like”
Pseudocyst Findings: Pseudocysts as small as 2–3 mm can be accurately detected by IOUS. They appear as well-defined hypoechoic masses with associated posterior enhancement and can contain debris of mixed echogenicity (Fig. 12.15). Ultrasonography can help to differentiate pseudocysts from abscesses (less well-defined cystic masses with mixed echogenicity and/or presence of luminal gas), hematomas (mixed echogenicity, fluid-fluid levels suggesting clot), or malignancy (intraluminal nodules and/or irregular pseudocyst wall) [17]. CEUS has a 100 % sensitivity and specificity for characterizing pseudocysts, which appear as a non-enhancing lesion in all phases with a nonvascular core. However, traversing vessels may be found in the early stages [18, 19].
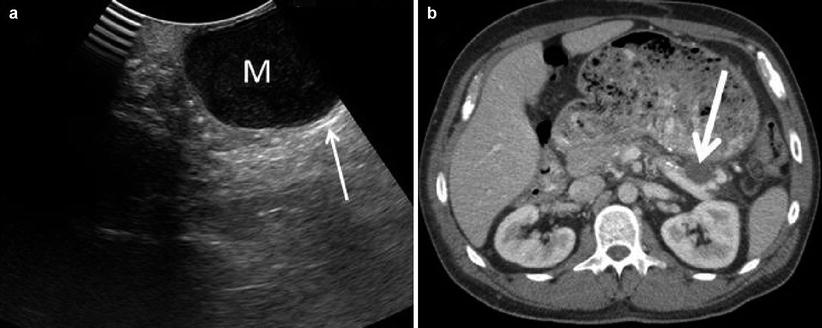

Fig. 12.15
Pseudocyst – (a) The typical pseudocyst (M) will appear well circumscribed and uniformly hypoechoic with posterior enhancement (thin white arrow). The corresponding CT scan (b) shows the pseudocyst (thick white arrow)
Pseudoaneurysm Findings: The development of a pseudoaneurysm involving a peripancreatic vessel is a known complication of pancreatitis and can be fatal if it ruptures. IOUS with color Doppler can assist localization of the lesion, identify the extent of the vessel involvement, and help gain proximal and distal control prior to exposure.
Pancreatic Cysts
Indications: Intraoperative ultrasound plays an integral part in the management of cystic lesions of the pancreas, particularly the characterization of suspected intraductal mucinous neoplasms (IPMNs). The malignant potential of IPMNs is directly related to its relationship with the main pancreatic duct. Main branch or mixed subtypes have a mean invasive malignancy rate of 43 % and should be resected. The side-branch subtype has a lower associated mean invasive malignancy rate of 17 % and is recommended for selective resection or enucleation based on the “Sendai criteria.” Included in these criteria are lesions greater than 3 cm and those that are clinically symptomatic or have high-risk features (main duct involvement, thickened cyst wall, mural nodules, positive cytology, main duct size 5–9 mm, or abrupt change in caliber of pancreatic duct with distal pancreatic atrophy) [20]. Each of these features is identifiable by ultrasound. IOUS has been shown to be more sensitive than, and equally specific as EUS or CT for the diagnosis of IPMN, with improved ability to assess the extent of ductal involvement [21]. If there is no suggestion of main duct involvement, IOUS may be utilized to determine the extent of the resection required. Recent studies have shown that enucleation for solitary cystic lesions not involving the main duct may be a viable option for resection [22, 23]. IOUS is an important tool for safely performing localized resection of small lesions, as it can delineate surrounding vessels and ducts. Anatomic proximity of a cyst to the main pancreatic duct may influence the decision to enucleate versus resect because of the risk for pancreatic fistula. Cysts that are less than 2 mm from the main pancreatic duct have a risk of pancreatic fistula development nearing 60 %, whereas those more than 2 mm from the main pancreatic duct are associated with a 19 % incidence of fistulization [24]. Intraoperative ultrasound may also be useful to characterize non-IPMN cyst anatomy or assist in obtaining aspirates for diagnosis [25, 26]. However, as most of this can now be done via EUS preoperatively, the role of IOUS is to delineate anatomy for resection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







