Robotic (Intuitive Surgical, Sunnyvale, CA, USA)
Laparoscopic
Three 8 mm robotic ports
Two 12 mm ports
Hot Shears™ (Monopolar Curved Scissors)
One 15 mm port
Maryland™ Bipolar Forceps
One 5 mm port
Cadiere™ Forceps
Suction
Large needle driver
Grasper
ProGrasp™ Forceps
Kelly
Basic accessory kit and drapes
Scissors
Intuitive surgical camera head
Ligasure™ (Valleylab, Boulder, CO, USA)
Intuitive surgical 0° and 30° endoscopes
16 mm stapler (Endo-GIA™/Covidien Corp. Dublin, Ireland)
Large and small clip appliers (Hem-o-lok®/Weck Closure Systems, Research Triangle Park, NC, USA, Lapra-Ty®/Ethicon Endo-Surgery, CA, USA)
Three specimen bags
Ligaloop strings (Braun-Dexon, Spangenberg, Germany)
Patient Positioning
After induction of general endotracheal anesthesia, a nasogastric tube and an 18 Ch Foley urinary catheter are inserted. The patient is placed in lithotomy position with arms adducted and padded. The legs are also abducted and slightly lowered on spreader bars. The table is placed in 25° Trendelenburg position during the RC and PLND. For the urinary diversion the Trendelenburg position is decreased to 10–15°.
Trocar Configuration
Port placement is critical for successful robotic surgery. A six-port technique is used with the camera port placed 5 cm above the umbilicus in the midline (Fig. 12.1). The camera port is placed with the Hasson technique and the other ports are placed with direct view. During the port placement, a pressure of 18 mmHg can be helpful in creating additional tension on the abdominal wall. Two robotic ports are placed symmetrically in the level of the umbilicus on the left and right side, lateral to the rectus sheath, A third robotic port is placed just above and medial to the left anterior superior iliac spine through a 15-mm port, thereby enabling laparoscopic stapling by the assistant when the third robotic port is temporarily disconnected. This is the so-called hybrid port. Two assistant ports are placed on either side of the right robotic instrument port.
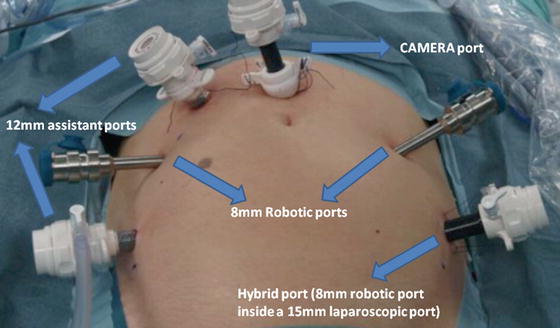

Fig. 12.1
Trocar configuration for robot-assisted radical cystectomy
Surgical Technique
It is crucial that the console surgeon follows accurately and always with the same order the steps of the operation in order to have the best results and reduce operating time. The following steps have been refined in our institution many times to achieve the optimum result [9, 10]. All the steps are summarized in Table 12.2. Steps 5–13 concern the urinary diversion with the formation of the ileal orthotopic neobladder.
Table 12.2
Robot-assisted radical cystectomy (RARC) with orthotopic ileal neobladder formation “step by step”
1. Dissection of both ureters along the ureterovesical junction |
2. Cystoprostatectomy (males) |
(a) Posterior dissection of vasa deferentia and seminal vesicles |
(b) Enter Retzius space and bladder drop of the right side |
(c) Incision of the right endopelvic fascia |
(d) Ligation of right vesical pedicles with Ligasure |
(e) Intrafascial sparing of the right neurovascular bundle |
(f) Repeat (b), (c), (d), (e) on the left side |
(g) Closure of the urethrovesical junction with a suture |
(h) Apical dissection with preservation of an adequate urethral stump |
(i) Completion of cystoprostatectomy or anterior pelvic exenteration (females) |
Resection of bladder, urethra, uterus, cervix, ovaries, anterior wall of the vagina (vagina preservation when needed) |
3. Extended lymph node dissection |
4. Transposition of the left ureter through the sigmoid mesentery |
5. Reduction of Trendelenburg tilt to 10–15° from 25° |
6. Entero-urethral anastomosis |
7. Isolation of 50 cm of distal ileum for Studer neobladder and stapling |
8. Detubularization of the ileal segment |
9. Suturing of the posterior wall of the pouch |
10. Folding of the reservoir |
11. Construction of the ureteral Wallace plate, stenting of both ureters through the afferent limp of the Studer pouch |
12. Entero-ureteral anastomosis |
13. Closure of the remaining reservoir |
14. Placing a drain in the small pelvis |
Entero-Urethral Anastomosis
The patient is placed in reduced 10–15° Trendelenburg position. The 0° lens is used for this initial step. The ileum is sufficiently mobilized in order to reach down to the urethra. Using robotic scissors, a 20 Ch opening (Fig. 12.2) is made to the antimesentric site of the ileum, which is isolated between two Ligaloop strings (Braun-Dexon, Spangenberg, Germany), passed around the intestine. The anastomosis is performed according to the Van Velthoven technique with a two-times 16 cm running 4-0 Quill™ suture, allowing for 10–12 stitches (Fig. 12.3). A needle driver and a Cadiere forceps are used for the above maneuvers. It is important previously to preserve an adequate urethral stump and have a silicone catheter placed.
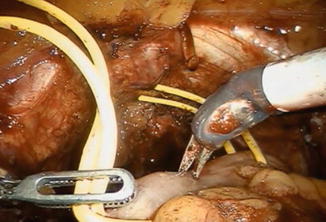
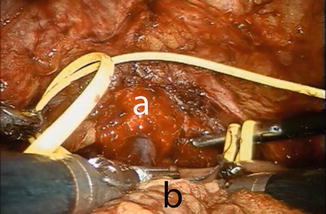

Fig. 12.2
A 20 Ch opening is made using robotic scissors to the respective site for the urethra-ileal anastomosis, which is being mobilized using two Ligaloop strings

Fig. 12.3
Urethro-ileal anastomosis using the VanVelthoven technique. a urethral stump, b ileal loop
Isolation of 50 cm Ileum
The orthotopic neobladder is fashioned with the Studer technique from a 50 cm segment of terminal ileum. The intestine is isolated using laparoscopic 60 mm intestinal stapler (Endo-GIA; Covidien Corp., Dublin, Ireland) (Fig. 12.4). The stapler is inserted by the bedside surgeon, using the hybrid 15 mm port. The ileum is stapled 40 cm proximal to the urethro-ileal anastomosis. The continuity of the small bowel is restored by using the same stapler, positioning the distal and proximal end of the ileum side to side with the antimesentery parts facing each other. An additional transverse firing of the Endo-GIA staple is used to secure the open ends of the ileal limbs.
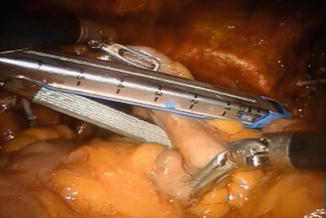

Fig. 12.4
Isolation of 50 cm of ileum for the neobladder. The ruler of the stapler can facilitate measurements. All maneuvers are performed using two Cadiere forceps
Detubularization
The distal 40 cm of the isolated ileal segment are detubularized along the antimesenteric border with cold scissors (Fig. 12.5), leaving a 10 cm intact proximal isoperistaltic afferent limb.
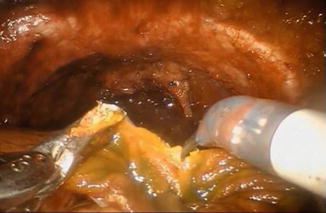

Fig. 12.5
Detubularization of the ileal segment
Formation of Studer Neobladder
The posterior part of the Studer pouch is closed using multiple running sutures (15 cm 3-0 V-Loc™) in a seromuscular fashion, avoiding suturing the mucosa (Fig. 12.6). After the posterior part is sutured, the distal half of the anterior part of the reservoir is sutured, using the same suture. The 0 or 30° lens can be useful for this part of procedure. The proximal half of the anterior part of the reservoir is left open and is closed in the last part of the procedure.
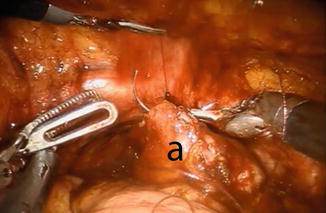

Fig. 12.6
Suturing the posterior wall (a) of the reservoir. Observe the stay suture held by the fourth robotic arm
Uretero-Enteral Anastomosis
The anastomosis between the ureters and the afferent limb is performed using the Wallace technique using a 0 lens (Fig. 12.7). A 3-0 Biosyn® stitch is placed at the distal end of each ureter. The left ureter is transposed to the right side by creating a tunnel under the sigmoid mesentery, just below the inferior rectal artery. The ureters are then spatulated 2 cm. The posterior walls of ureters are sutured side to side, using a 15 cm 4-0 V-Loc™ suture. Before commencing the anastomosis, two single-J 40 cm ureteric stents are introduced via the Seldinger technique through two separate 4 mm incision in the midline just above the pubic symphysis. The stents are pulled through the afferent limb (Fig. 12.8) and pushed up in to the ureters on each side, using the Cadiere forceps. The Wallace plate is sutured to the afferent limb of the Studer reservoir, using a 16 cm 4-0 Quill™ suture. After the uretero-enteral anastomosis is completed, the stents are sutured and fixed to the skin.
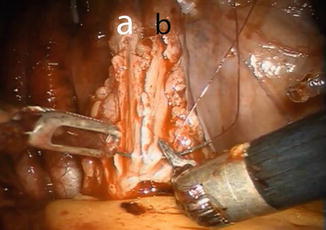
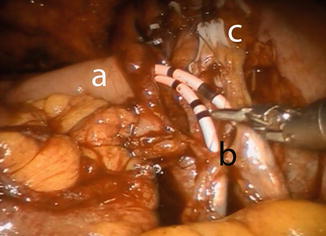

Fig. 12.7
Construction of the Wallace ureteral plate. Left (a) and right ureter (b) are spatulated 2 cm and tacked in place using a preplaced clip

Fig. 12.8
Uretero-ileal anastomosis. Stents are already placed in both ureters using guidewires. The stents are first passed through the afferent limp of the Studer reservoir. (a) afferent limp, (b) Wallace plate, (c) clips at the distal ends of both ureters for obtaining frozen sections
Closure of the Studer Reservoir
The remaining part of the reservoir is then closed with a running 3-0 V-Loc™ suture, using a 0° lens (Fig. 12.9). The balloon of the indwelling catheter is filled with 10 cm3. The neobladder is then filled with 50 cm3 of saline to check for leakage. If leakage is observed, extra sutures will have to be considered. A 21 Ch passive drainage is introduced and placed in the small pelvis (Fig. 12.10).
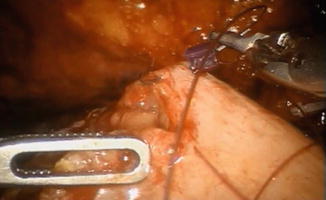
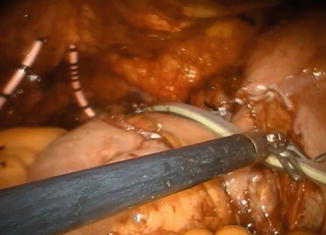

Fig. 12.9
Closure of the remaining anterior part of the pouch

Fig. 12.10
Final intraoperative image. Drain is placed into the small pelvis
Postoperative Care
Careful manual irrigation of the reservoir has to be performed every 6–8 h to avoid catheter blockage by mucus overproduction. The drain can be removed when the drainage is <200 cm3. The ureteral stents will be removed 1 week postoperatively. The urethral catheter can be removed approximately 3–4 weeks after the operation, without retrograde cystography. Pain management is achieved with the administration of 10 mg oxicodon b.i.d, 1 g paracetamol q.i.d., and parenteral morphine when required.
The patient is meticulously informed after the removal of the urethral catheter about the method and frequency of urination.
Complications and Management
RARC with neobladder formation remains a high-risk procedure with complications rate ranging from 30 to 50 % [11]. The rate is increasing due to the fact that RARC is being used more often in the elderly population, since the incidence of muscle-invasive bladder cancer peaks in the octogenarians [12]. Recent studies have shown though that even in the elderly patients, results can be similar to the ones of the younger patients [13, 14]. On the other hand, increasing experience, refinement, and standardization of the procedure continuously lowers the complication rates.
Table 12.3 shows the incidence of the short- and long-term complications that are related to the neobladder diversion in general [15–17]. These complications do not differ from the ones occurring in the open procedure. Short-term complications are reported within the first 90 days postoperatively. All complications are graded according to the CLAVIEN classification system, as revised by Dindo [18]. Each respective management is also depicted.
Table 12.3
The incidence of the short- and long-term complications that are related to the neobladder diversion
Incidence (%) | CLAVIEN grade | Management
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|


