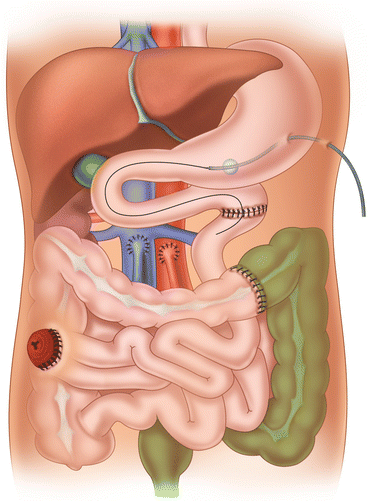
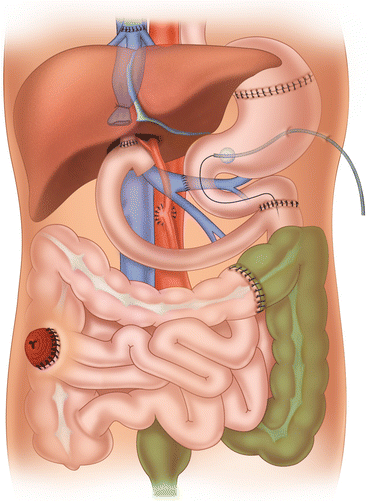
Fig. 25.1
Graft types: (a) isolated intestinal graft; (b) combined liver-intestinal graft; (c) multivisceral graft
25.3 Graft Types and Indications
The use of the three main types of intestinal graft has changed over time, depending on the experience of the surgical team and the change in indications for intestinal transplantation. A paper published in 2009 examined the shifting of graft types over time in a large cohort (500 patients) of intestinal transplants performed over an 18-year period [4]. Dividing the 18-year experience in intestinal/multivisceral transplantation into three eras, Era I (1990–1994), Era II (1995–2001), and Era III (2001–2008), the indications rose from 35 % in Era I to 46 % in Era III for isolated intestinal transplants and from 20 % in Era I to 34 % in Era III for multivisceral transplantation. On the contrary, liver-intestinal transplants (type II) decreased from 45 % (Era I) to 20 % (Era III) with prevalent indications in pediatric patients. The polarization of the intestinal grafts in isolated intestine and multivisceral transplant reflects an almost international attitude and is due to early referrals of irreversible intestinal failure patients to transplant centers (isolated intestinal transplants) and the technical complexity of the liver-intestinal transplant compared to multivisceral transplantation.
25.4 Isolated Small Bowel Transplantation
The main indication for the transplant of the isolated midgut is any intestinal irreversible failure with normal liver function and no necessity to replace the stomach and the duodenum secondary to anatomical damage or dysmotility of these two organs. A variety of diseases can be treated with this transplant in adults and children: congenital or acquired anatomical shortening of the small bowel, diffuse dysmotility or congenital intestinal villi dysfunction, and desmoid tumors (see also Chap. 24). The small bowel can be transplanted alone or in continuity with the right colon to provide better absorption; in a few patients, the intestinal graft was transplanted with preservation of the enteric nervous ganglia, but no data exist on the outcome of ganglia preservation. The isolated intestinal graft can be transplanted reducing its size in the case of a small residual abdominal cavity, and of course it can be a perfect type of graft if there is an indication for living related transplantation, particularly from adult to child.
25.4.1 Recipient Procedure
After careful laparotomy, a total enterectomy of the recipient’s residual small bowel is performed, if needed. The small bowel graft with or without the right colon can be revascularized orthotopically or heterotopically. In the first case, the SMA is anastomosed end-to-end to the recipient’s SMA with 7-0 Prolene, and the same is done with the SMV of the donor and recipient. In the case of previous recipient SMA disease, the arterial inflow should be obtained with an interposition arterial graft from the recipient aorta and then with an anastomosis between the donor SMA and the arterial graft (Fig. 25.2). An orthotopical variant of the venous outflow is to anastomose the donor superior mesenteric vein end-to-side to the recipient portal vein. This procedure is sometimes more difficult than the others and requires an extended mobilization of the recipient’s pancreas and duodenum and exposure of the portal vein without damaging the recipient bile duct [5].
In the case of difficult dissection of the superior mesenteric vein or previous thrombosis of the recipient SMV and if the surgeon does not want to perform an orthotopic SMV reconstruction with the portal vein as described previously, a heterotopic revascularization of the intestinal graft can be performed (Fig. 25.3). In this case, after the enterectomy of the residual intestine, the surgeon should expose the anterior and lateral walls of the infrarenal aorta and inferior vena cava. After partial clamping of the aorta and IVC, an arterial graft and a venous graft from the donor are anastomosed end-to-side to the recipient’s large abdominal vessels. Finally, first the donor SMA is anastomosed to the arterial graft followed by the anastomosis between the donor SMV and the vein graft. It should be emphasized that the heterotopical revascularization of the intestinal allograft facilitates its implantation. However, special attention should be paid to avoid kinking of the artery and vein by excessive length of the two vessels.
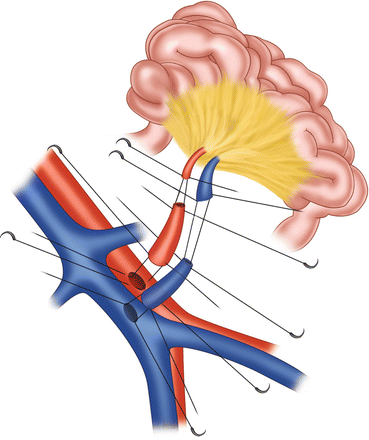

Fig. 25.3
Eterotopic revascularization of the intestinal graft
At the time of the initial clinical experience in small bowel transplantation, there was concern about possible native liver dysfunction in using this heterotopical venous reconstruction. Later, it was observed that reconstruction of the donor superior mesenteric vein into the systemic circulation did not cause any liver dysfunction and could be considered as effective as direct reconstruction into the portal venous system [6].
Tzakis et al. [7] first described the inclusion of the right colon in the isolated small bowel transplant graft. The supposed advantage of including the right colon was to improve the electrolytes and water absorption by the graft. Initial concerns were the possible increase in rejection of the colon segment and the subsequent increase in mortality rates of the recipients. In the first review of the results of such composite grafts done by the International Intestinal Transplant Registry in 2007, patients transplanted with isolated intestine together with the colon showed a significantly lower requirement of i.v. fluids or parenteral nutrition early and later after transplantation. Furthermore, no significant differences were reported in terms of mortality or rejection when intestine plus colon was compared to small bowel graft alone.
Once the graft has been revascularized and hemostasis obtained, the intestinal continuity is reconstructed proximally with the fourth portion of the duodenum or the stomach of the recipient. Distally the small bowel or the right colon is reconstructed with the remaining left colon or sigmoid colon. In some conditions, an endorectal pull-through with sphincter preservation and rectal mucosectomy is indicated. This quite difficult procedure can be indicated in cases of multiple juvenile polyposis extended into the rectum or in the case of Hirschsprung’s disease with diffuse dysmotility extended from the rectum to the jejunum [7]. After bowel reconstruction, the surgery ends with the feature of the terminal ileostomy, having first placed an enteric feeding tube into the intestinal allograft. The distal stoma is necessary in order to obtain easy access to the intestinal allograft for intestinal biopsy and endoscopy. Some centers also advocate creating a proximal stoma to improve the immunological monitoring of the graft also for enteral nutrition. Of course, depending on personal choices, it is better to remember that stomas need to be closed at some time, and stoma closure is not without complications in such patients. The distal stoma is without question an extreme need for immunological monitoring; for differential diagnosis during the follow-up endoscopies, it is important to observe the bowel mucosa and perform biopsies of the allograft intestine and of the native bowel. Nutrition of the allograft can be obtained placing a transgastrostomy feeding tube long enough to reach the lumen of the allograft intestine.
25.5 Combined Liver-Intestine Transplantation
This composite graft, together with isolated intestinal transplantation, was one of the first models of intestinal transplant performed. The principal indications consist of irreversible intestinal failure combined with cholestatic cirrhosis with portal hypertension. The main patient population which may require this type of allograft is pediatric. The combination of a liver-intestine allograft invariably requires the sparing of the native stomach-pancreas and duodenum with preservation of their vascular supply through the native celiac axis and superior mesenteric artery and providing the venous outflow through a portacaval shunt to be performed before vascular anastomosis of the composite graft. Due to the fact that this type of allograft was used more often in children, several kinds of reduced size grafts have been performed.
25.5.1 Recipient Procedure
After removal of the residual small bowel and colon, the total native hepatectomy is performed with the piggyback technique. After the dissection of the native bile duct, the hepatic artery is cut distal to the gastroduodenal artery. The portal vein is clamped and cut up into the hilum. At this point, with the liver devascularized, the native proximal portal vein is anastomosed to the native inferior vena cava in end-to-side fashion with running sutures and unclamped to avoid congestion of the native gastro-pancreatic-duodenal bloc. After complete mobilization of the native liver, hepatectomy is performed after clamping of the hepatic vein confluence.
The liver-intestine allograft is then placed on the surgical field, and the first arterial reconstruction is performed. This can be done anastomosing the donor aortic patch on the anterior wall of the recipient’s infrarenal aorta or, more easily, anastomosing first a segment of the donor’s thoracic aorta end-to-side to the anterior wall of the infra-renal recipient aorta, followed by the anastomosis of the aortic carrel patch including the takeoff of the donor celiac axis and SMA to the aorta conduit in end-to-end fashion with running sutures. There are two main pitfalls in this kind of arterial reconstruction: (a) if the aortic patch around the donor SMA is too short, the inferior wall of the aorta conduit may partially close the takeoff of the SMA; (b) if the aortic conduit is too long, this can kink, producing a critical arterial flow to the SMA or both SMA and celiac axis.
After completion of the arterial reconstruction, the liver is put in place in the right sub-diaphragmatic space, and the venous outflow is reconstructed, anastomosing the donor inferior caval vein to the confluence of the recipient’s hepatic veins. Only at this point is the liver-intestine allograft reperfused through the arterial reconstruction. Once complete hemostasis has been obtained, the intestinal continuity is performed, anastomosing first the proximal donor jejunal loop to the donor bile duct, followed by the anastomosis between the native fourth portion of the duodenum to the allograft jejunum and, finally, by the anastomosis between the native colon and the allograft last ileal loop just proximally to the segment of donor ileum which can be exteriorized for the temporary ileostomy (Fig. 25.4). A jejunal feeding tube is placed through a gastrostomy and pushed into the allograft jejunum through the duodenal-jejunal anastomosis.
25.5.2 Pitfalls of the Liver-Intestine Transplant Technique
Besides the arterial pitfalls described before which are rare but possible, there are two other major pitfalls in this procedure which we should be aware of. The first and the most frequent is the biliary tract reconstruction. Biliary complications secondary to the biliary-jejunal anastomosis are similar in rate to what one should expect after roux-en-Y biliary reconstruction in pediatric liver transplantation. The major concern is that in over-immunosuppressed patients, like intestinal transplant patients, this can become a life-threatening complication if not diagnosed promptly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree