Proper integration of surgery and systemic therapy is essential for improving outcomes in renal cell carcinoma (RCC). There is no current role for adjuvant therapy after nephrectomy for clinically localized disease. The potential benefits of neoadjuvant therapy for locally advanced nonmetastatic disease are in need of further study. In metastatic disease, the proper integration of cytoreductive surgery and systemic therapy remains to be elucidated. Presurgical targeted therapy is feasible and may be beneficial. Pending the results of randomized controlled trials, upfront cytoreductive nephrectomy in appropriate patients will likely continue as the paradigm of choice in metastatic RCC.
An estimated 58,240 people in the United States were diagnosed with kidney cancer in 2010, and 13,040 died of the disease. Forty percent of patients have regionally advanced or metastatic disease at diagnosis, and 10% to 28% develop recurrence or metastasis after surgery for localized disease. To maximize clinical outcomes, proper integration of surgery and systemic therapy is essential. This review focuses on the role of adjuvant therapy for renal cell carcinoma (RCC), neoadjuvant therapy for locally advanced disease, and multimodal therapy for metastatic RCC (mRCC), including cytoreductive nephrectomy and presurgical targeted therapy.
Nomenclature
The nomenclature of integrated systemic and surgical therapy is not standardized. For clarity, and as described in a recent collaborative review, the term adjuvant therapy is used in this review to describe treatment that is administered after complete surgical resection with the goal of reducing risk of recurrence in a patient without evidence of disease. Neoadjuvant therapy refers to use of a therapy before surgical resection of clinically localized disease. Presurgical therapy designates the administration of a therapy before planned cytoreductive surgery in mRCC.
Adjuvant therapy for RCC at increased risk of relapse
Despite the increase in early detection in the modern era, 10% to 28% of patients develop recurrence or distant metastasis after nephrectomy for clinically localized disease. Cure is elusive for patients with distant metastatic disease, with 5-year survival rates of approximately 10%. A wide range of adjuvant therapies have been investigated to reduce risk of recurrence. An ideal adjuvant therapy has a favorable toxicity profile, proven activity in metastatic disease, proven efficacy against the standard of care (observation) in phase 3 randomized trials, and can be administered on an outpatient basis to patients who are most likely to benefit from adjuvant therapy. Although no agents have reliably met these goals and observation remains the standard of care, trials of several promising therapies are in progress.
Defining Risk of Recurrence
A recognized disadvantage of the adjuvant approach is that some patients who have been cured with surgery alone are unnecessarily treated with adjuvant systemic therapy. An essential component of developing effective adjuvant therapy is to identify the population of patients who are at high risk of recurrence and thus most likely to benefit from adjuvant therapy.
Models incorporating clinical and pathologic data
Several prognostic models have been developed that predict the risk of progression after surgery for localized RCC using clinical and pathologic variables ( Table 1 ). These models, some of which have been externally validated, may prove to be useful for selecting patients for adjuvant therapy. Each model has limitations. For instance, Kattan and colleagues acknowledged that predicting 5-year recurrence-free survival limits the usefulness of their nomograms, because it does not capture the 15% to 19% rate of recurrence beyond 5 years. In addition, the 2001 Memorial Sloan-Kettering Cancer Center (MSKCC) nomogram included both clear cell and nonclear cell histology. The investigators thus chose to exclude nuclear grade from their nomogram because of controversy regarding the grading of nonclear cell tumors. Given the association between grade and outcome, the impact of excluding nuclear grade on the predictive capacity of the nomogram has been questioned. The 2005 MSKCC nomogram addresses this concern by limiting the model to clear cell RCC (ccRCC), which allowed nuclear grade to be included as a predictive variable.
| Author | Year | Institution | Type | Study Population (n) | Inclusion | Variables | Outcome | Timepoint (y) | Concordance Index | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNM | Histology | Nephrectomy | Years | Symptoms | Performance Status | Tumor Size | pT | pN | Grade | Histology | Necrosis | Vascular Invasion | ||||||||
| Kattan et al | 2001 | MSKCC | Nomogram | 601 | T1–3c, N0/x, M0 | Papillary, chromophobe, ccRCC | Partial, radical | 1989–1998 | ✓ | ✓ | ✓ (1997) | ✓ | Recurrence-free survival a | 5 | 0.74 | |||||
| Zisman | 2002 | UCLA | Algorithm (low, moderate, high risk) | 468 | T1–4, N0, M0 | Any | Partial, radical | 1989–2000 | ✓ | ✓ (1997) | ✓ | ✓ | Overall survival, disease-specific survival, local recurrence-free survival, and systemic recurrence-free survival | 1, 2, 3, 4, 5 | NA | |||||
| Leibovich et al | 2003 | Mayo | Algorithm (score between 0 and 11) | 1671 | T1–4, Nx –N2, M0 | ccRCC | Radical | 1970–2000 | ✓ | ✓ (2002) | ✓ | ✓ | NA | ✓ | Metastasis-free survival | 1, 3, 5, 7, 10 | 0.82 | |||
| Sorbellini et al | 2005 | MSKCC | Nomogram | 701 | T1–3c, N0/x, M0 | ccRCC | Partial, radical | 1989–2002 | ✓ | ✓ | ✓ (2002) | ✓ | NA | ✓ | ✓ | Recurrence-free survival a | 5 | 0.82 | ||
The modified University of California at Los Angeles (UCLA) Integrated Staging System (UISS) has been externally validated. Like the MSKCC nomograms, the UISS timeframe is limited to 5 years. A limitation of the UISS is that it assigns patients to low-risk, medium-risk, or high-risk groups rather than predicting risk for an individual patient. A range of outcomes is expected within each risk category. The discriminating abilities of the 2001 MSKCC nomogram and the UISS were compared using a multicenter European cohort of 2404 patients. The concordance indices were 0.71 and 0.68 for the MSKCC and UISS models, respectively. The MSKCC nomogram improved discrimination of the UISS intermediate-risk category.
Additional predictive models exist that are based solely on preoperative variables such as gender, symptoms, and imaging findings, including necrosis, lymphadenopathy, and tumor size. The postoperative models, which include pathologic variables, discriminate better than preoperative models and are therefore more appropriate for selection of candidates for adjuvant therapy. Nonetheless, preoperative-only models may help select intervention versus active surveillance, and may prove useful for identifying patients for neoadjuvant therapy.
Models integrating molecular markers with clinical and pathologic data
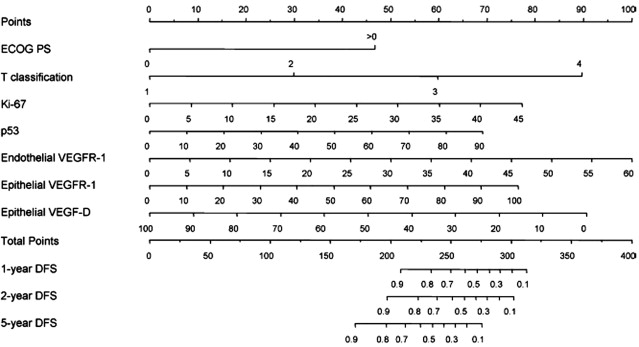
Future efforts to determine risk of recurrence after nephrectomy may incorporate molecular markers. For example, addition of data regarding expression of 3 molecular markers (carbonic anhydrase IX [CA IX], vimentin, and p53) to clinical markers (metastasis, T stage, performance status) yielded modestly improved accuracy in predicting disease-specific survival in localized and metastatic ccRCC compared with the UISS (concordance indices 0.79 vs 0.75). More recently, the same group published a nomogram combining clinical, pathologic, and molecular data to predict disease-free survival after nephrectomy for localized ccRCC ( Fig. 1 ). The nomogram variables include expression of Ki-67, p53, endothelial vascular endothelial growth factor receptor 1 (VEGFR-1), epithelial VEGFR-1, and epithelial VEGF-D along with T stage and performance status. The predictive ability of the 5 molecular markers alone exceeded that of the UISS (concordance index 0.84 vs 0.78). The accuracy of the nomogram incorporating the clinical, pathologic, and molecular data was higher still (concordance index 0.90).
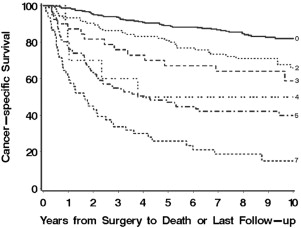
Similarly, the group from Mayo Clinic used immunohistochemistry to characterize expression of B7-H1, survivin, and Ki-67 in 634 patients treated with radical or partial nephrectomy for localized or metastatic ccRCC. The 3 molecular markers were each shown to be independently associated with RCC-specific death. Weighted scores were assigned to marker expression, which was dichotomized. The total score (range 0–7), termed BioScore, was able to discriminate cancer-specific survival ( Fig. 2 ). The investigators showed that the addition of BioScore improved the predictive ability of other models including TNM staging (concordance index 0.82 vs 0.79) and the UISS (0.82 vs 0.77).
Predictive models that include biomarkers are promising and may help select patients for adjuvant therapy. These models require independent validation and standardization of laboratory techniques before incorporation into clinical practice. Moreover, the costs of using biomarkers must be considered in context of the thus far modest improvement over the user-friendly, readily available clinicopathologic models.
Reported Adjuvant Trials
Radiotherapy
Initial adjuvant studies focused on improving local control with radiotherapy. Radical nephrectomy provides excellent local cancer control and recurrence of RCC is typically distant from the primary. Because local failure is uncommon, little role is expected for adjuvant radiotherapy. The data support this expectation.
In a prospective trial conducted from 1961 to 1970 at Newcastle General Hospital (Newcastle Upon Tyne, UK), patients with a completely resected primary tumor and no evidence of metastatic disease were randomized to observation (n = 49) or adjuvant radiation to the renal bed, incision, and para-aortic nodes (n = 51). There was no significant difference in local recurrence, development of metastases, or survival. Significant side effects, including 4 deaths from liver failure, were attributed to the radiation.
In 1987, Kjaer and Frederiksen reported the results of a multicenter randomized trial in Copenhagen, Denmark. Between 1979 and 1984, patients with stage II and III RCC were randomized after nephrectomy to 50 Gy of external beam radiotherapy in 20 fractions to the kidney bed and nodes (n = 32) or observation (n = 33). Radiotherapy was associated with hepatic, gastric, and duodenal injury, but no improvement in relapse. In 19% of patients, radiotherapy complications contributed to the patient’s death.
Hormonal therapy
Some renal tumor cells express glucocorticoid receptors that can be blocked by hormonal agents, such as medroxyprogesterone acetate (MPA), which have reported activity in the metastatic setting. In 1987, Pizzocaro and colleagues reported a multicenter trial in which patients were randomized to 1 year of adjuvant MPA (n = 58) or observation (n = 62) after radical nephrectomy for nonmetastatic RCC. Sixty-two (51%) of the patients had at least T3 disease. After a median follow-up of 5 years, rates of relapse were similar in the intervention and control groups (32.7 vs 33.9%). Complications were common in the intervention group.
Immunotherapy
There is a strong rationale for immunotherapy in RCC. The essential role played by the host immune system is shown by case reports of spontaneous regression of metastatic disease after nephrectomy or ablation of the primary tumor, as well as the presence of tumor-infiltrating immune cells in nephrectomy specimens, which have shown antitumor activity. The primary tumor is believed to have an immunosuppressive effect that can be ameliorated by nephrectomy. The aim of immunotherapy is to augment the host immune response. Once the immune sink has been excised, it is posited that adjuvant immunotherapy can better treat the remaining subclinical disease that leads to recurrence. Various methodologies have been used, including administration of cytokines, vaccines, dendritic cell therapy, and allogeneic hematopoietic stem-cell transplant to take advantage of graft-versus-tumor effect.
An aspect of the antitumor immune response is believed to be mediated by CD8+ cytotoxic T lymphocytes and amplified by CD4+ helper T cells, which secrete cytokines including interleukin 2 (IL-2) and interferon α (IFN-α). Exogenous IL-2 and IFN-α have shown efficacy in metastatic disease, with response rates up to 20% and a 5% durable complete response for IL-2. Based on these findings, several randomized trials investigated IL-2 and IFN-α as adjuvant therapy but were unable to show a disease-free or overall survival benefit ( Table 2 ). In 1 trial, adjuvant chemoimmunotherapy was associated with worse 5-year overall survival when compared with control (58 vs 76%, P = .028).
| First Author | Year | Eligibility | Intervention | Control | N | Median Follow-up | Primary End Point | Outcome (Intervention vs Control) | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Pizzocaro et al | 2001 | Robson II or III | IFN-α | Observation | 247 | NA | 5-year OS | 66.5% vs 66.0% | .861 |
| Messing et al | 2003 | pT3–4a or N+ | IFN-α | Observation | 283 | 10.4 years | Median OS | 5.1 vs 7.4 years | .09 |
| Clark et al | 2003 | pT3b–4 or N+ or M1 (resected) | High-dose IL-2 | Observation | 69 | 22 months | 2-year DFS | 48% vs 55% | .431 |
| Atzpodien et al | 2005 | pT3b–4 or N+ or M1 (resected) | IFN-α + IL-2 + 5-fluorouracil | Observation | 203 | 4.3 years | 5-year OS | 58% vs 76% | .028 |
Adjuvant active specific immunotherapy with vaccines has also been used, with mostly unfavorable results. In 1996, Galligioni and colleagues reported a trial in which patients after nephrectomy were randomized to observation (n = 60) versus intradermal injection of irradiated tumor cells and Bacille Calmette-Guérin (n = 60). One month after completing therapy, a delayed-type cutaneous hypersensitivity reaction to autologous tumor cells was shown in 70% of immunized patients. Despite this induced tumor-specific immunoreactivity, 5-year disease-free survival was similar in the intervention and control arms (63 vs 72%, P = nonsignificant).
In 2004, Jocham and colleagues reported the only successful adjuvant trial in RCC to date. In 1997 to 1998 at 55 centers in Germany, 558 patients scheduled for radical nephrectomy were enrolled. Patients were randomized before nephrectomy to receive 6 adjuvant intradermal injections of autologous tumor vaccine at 4-week intervals or observation. After nephrectomy, only patients with pT2 to 3b, pN0 to 3, M0 RCC and Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2 were permitted to continue in the trial. Patients with pT1 or pT4 disease were excluded. Tumor cells were devitalized by rapid freezing at −82°C and thawing. The primary end point was tumor progression.
Five patients withdrew consent before surgery. After surgery, 174 patients were withdrawn from the study for various reasons such as non-RCC histology, incorrect tumor stage, and inability to prepare the vaccine. More of these patients were in the vaccine arm (n = 99 vs 75). Among the remaining 379 patients, 5-year progression-free survival was higher in the vaccine group (77.4 vs 67.8%, P = .02). At 5 years, the hazard ratio for progression was 1.58 (95% confidence interval [CI] 1.05–2.37, P = .02) in favor of vaccination. The 5-year progression-free survival benefit favoring vaccination was pronounced in patients with pT3 disease (67.5 vs 49.7%, P = .039). Twelve adverse events of mild to moderate severity were reported in the vaccine group.
The trial was criticized for the large loss of patients (32%) after randomization, which was imbalanced between study arms. Given the study design, in which patients were randomized before pathologic diagnosis and staging, some loss of patients was inevitable. A secondary intention-to-treat analysis was subsequently reported in abstract form with a larger number of patients in the vaccine (n = 233) and control (n = 244) groups. The vaccine was still associated with improved progression-free survival ( P = .048). The magnitude of the benefit was not reported. There was no difference in overall survival ( P = .12). A retrospective matched-pair analysis of the same vaccine protocol in 495 patients was recently reported. With median follow-up of 131 months, the tumor cell vaccine was an independent predictor of overall survival on multivariable analysis in the whole group (hazard ratio [HR] 1.28, P = .030), as well as in the subset of patients with pT3 disease (HR 1.67, P = .011). Despite the progression-free survival benefit noted in a randomized trial and strong corroborating retrospective evidence, the therapy was not accepted broadly into practice, which led to the insolvency of the manufacturer of the vaccine.
In the largest phase 3 adjuvant trial in RCC, patients were randomized to receive vitespen (Oncophage) (n = 409) or observation (n = 409) after nephrectomy. Vitespen is a heat shock protein (HSP) vaccine. HSPs are intracellular chaperones that play a role in the loading of antigenic peptides onto major histocompatibility complex class I molecules, eliciting an immune response. Autologous HSP vaccines consist of HSP-peptide complexes, which are isolated from a patient’s tumor. In an intention-to treat analysis, the rate of recurrence was similar in the vitespen and control groups (37.7 vs 39.8%, P = .506) after a median follow-up of 1.9 years.
Other
Thalidomide, an antiangiogenic and immunomodulatory drug, has activity in mRCC. It has been investigated in the adjuvant setting in a single-institution trial. Patients with T2 (high-grade) to T4 or node-positive disease were randomized to thalidomide 300 mg daily for 2 years (n = 23) or observation (n = 23). The protocol was terminated early after a scheduled interim analysis showed that adjuvant thalidomide was unlikely to have the expected clinical benefit. Three-year recurrence-free survival was inferior in the thalidomide arm (28.7 vs 69.3%, P = .022). There was no difference in cancer-specific survival at 2 or 3 years.
Ongoing or Unreported Adjuvant Trials
Despite the host of negative adjuvant studies to date, there are numerous ongoing trials of adjuvant therapy using targeted agents, which are described in detail later. Five of the trials compare agents with proven activity in metastatic disease with placebo. ASSURE, S-TRAC, SORCE, and PROTECT compare adjuvant VEGF-targeted therapy with placebo, and EVEREST evaluates the role of adjuvant mammalian target of rapamycin (mTOR) inhibition. Although targeted agents might be effective in the adjuvant setting, some evidence suggests that they can adversely affect tumor biology, including promotion of tumor invasiveness and development of resistant metastases. All of the trials are thus targeted at patients with high risk of recurrence, some using the predictive models described earlier. Both SORCE and EVEREST include patients with nonclear cell histology.
ARISER
Girentuximab (Rencarex) is a chimeric monoclonal antibody against CA IX, also called the G250 antigen. CA IX is highly expressed on the cell surface of ccRCC. Girentuximab binds CA IX and is posited to induce antibody-dependent cellular cytotoxicity through natural killer cells and other immune effector cells. A double-blind, placebo-controlled phase III trial, Adjuvant Rencarex Immunotherapy Trial to Study Efficacy in Nonmetastatic RCC (ARISER), was undertaken to evaluate the impact of adjuvant girentuximab on the disease-free and overall survival of patients with ccRCC at high risk of recurrence ( http://www.clinicaltrials.gov/ ; NCT00087022). A total of 864 patients with nonmetastatic high-grade T1b/T2, or T3-T4, or N+ ccRCC were randomized to weekly infusions of girentuximab or placebo for 24 weeks. Enrollment was completed in 2008. By January 2011, 340 recurrences had been reported. In November 2011, in face of a declining recurrence rate, the Independent Data Monitoring Committee recommended terminating the interim analysis in favor of completing the final analysis, which is expected to be published in 2012.
ASSURE
A phase III ECOG trial entitled Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma (ASSURE) has completed enrollment after enrolling more than 1900 patients ( http://www.clinicaltrials.gov/ ; NCT00326898). Sorafenib (Nexavar) is an oral small molecule inhibitor of several tyrosine kinases, including Raf-kinase and VEGFR. Sunitinib (Sutent) is an oral small-molecule multityrosine kinase inhibitor of VEGFR, platelet-derived growth factor receptor (PDGFR), and KIT. Both sorafenib and sunitinib are active in mRCC. Patients with nonmetastatic T1b (high-grade) to T4 or N+ RCC were randomized to up to 9 6-week courses of adjuvant sunitinib or sorafenib or placebo. The primary outcome is disease-free survival. Secondary end points include overall survival, safety, and molecular marker analyses. Although the study results are expected in April 2016, there has been concern regarding drug toxicity and early termination of therapy that may adversely influence trial outcome.
S-TRAC
Sunitinib is also being tested in an industry-sponsored phase III trial entitled Sunitinib Treatment of Renal Adjuvant Cancer (S-TRAC). Patients with high-risk ccRCC as defined by the UISS are being randomized to 1 year of sunitinib or placebo. Enrollment started in July 2007. Estimated enrollment is 720 patients, with an anticipated completion in July 2017. The primary end point is disease-free survival. Secondary end points include overall survival, safety, and patient reported outcomes ( http://www.clinicaltrials.gov/ ; NCT00375674).
SORCE
A Medical Research Council trial entitled A Phase III Randomised Double-Blind Study Comparing Sorafenib With Placebo in Patients With Resected Primary Renal Cell Carcinoma at High or Intermediate Risk of Relapse (SORCE) is ongoing in the United Kingdom ( http://www.clinicaltrials.gov/ ; NCT00492258). Patients with RCC at intermediate or high risk of relapse (Leibovich score 3–11) are randomized after nephrectomy to placebo or 1 year of sorafenib or 3 years of sorafenib. The primary outcome is disease-free survival, with secondary outcomes including metastasis-free survival, overall survival, cost-effectiveness, and toxicity. Anticipated enrollment is 1656 patients. The final data for the primary result are expected to be collected in August 2012.
PROTECT
An industry-sponsored trial referred to as a Study to Evaluate Pazopanib as an Adjuvant Treatment for Localized RCC (PROTECT), is in progress ( http://www.clinicaltrials.gov/ ; NCT01235962). Pazopanib (Votrient) is a potent multityrosine kinase inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, PDGFR, and c-kit that is associated with improved progression-free survival in locally advanced and mRCC. Patients with nonmetastatic T2 (high-grade) to T4 or N+ disease are being randomized after nephrectomy to 12 months of pazopanib or placebo. The primary outcome is disease-free survival. Overall survival is a secondary end point, along with safety and quality of life. Enrollment of the intended 1500 patients started in November 2010. The final data for the primary outcome will be collected in October 2015.
EVEREST
A Southwest Oncology Group (SWOG) trial called Everolimus for Renal Cancer Ensuing Surgical Therapy (EVEREST) is also currently enrolling participants ( http://www.clinicaltrials.gov/ ; NCT01120249). Everolimus (Afinitor) is an mTOR inhibitor that has been shown to improve progression-free survival in patients with mRCC that has progressed on sunitinib or sorafenib. Patients with nonmetastatic T1b (high-grade) to T4 or N+ RCC are randomized after nephrectomy to 9 courses of everolimus or placebo. The primary outcome is recurrence-free survival. Toxicity and overall survival are secondary end points. Enrollment of an anticipated 1218 patients started in 2011. The final data for the primary result are expected to be collected in August 2013.
Adjuvant Therapy in Current Practice
There are no strong data to support the use of adjuvant therapy after nephrectomy for clinically localized disease. In randomized controlled trials, adjuvant radiotherapy, MPA, IL-2, IFN-α and thalidomide all showed no impact on disease progression or survival. A single study, which has been criticized for methodological flaws, reported a large progression-free survival benefit with adjuvant autologous tumor vaccination. Other vaccine studies failed in the adjuvant setting. Ongoing studies of several tyrosine kinase inhibitors, an mTOR inhibitor, and a monoclonal antibody against CA IX are continuing the effort to decrease the risk of cancer recurrence and progression after nephrectomy for localized disease. The only accepted role for adjuvant therapy is in patients with high risk of recurrence in the setting of a research trial.
Adjuvant therapy for RCC at increased risk of relapse
Despite the increase in early detection in the modern era, 10% to 28% of patients develop recurrence or distant metastasis after nephrectomy for clinically localized disease. Cure is elusive for patients with distant metastatic disease, with 5-year survival rates of approximately 10%. A wide range of adjuvant therapies have been investigated to reduce risk of recurrence. An ideal adjuvant therapy has a favorable toxicity profile, proven activity in metastatic disease, proven efficacy against the standard of care (observation) in phase 3 randomized trials, and can be administered on an outpatient basis to patients who are most likely to benefit from adjuvant therapy. Although no agents have reliably met these goals and observation remains the standard of care, trials of several promising therapies are in progress.
Defining Risk of Recurrence
A recognized disadvantage of the adjuvant approach is that some patients who have been cured with surgery alone are unnecessarily treated with adjuvant systemic therapy. An essential component of developing effective adjuvant therapy is to identify the population of patients who are at high risk of recurrence and thus most likely to benefit from adjuvant therapy.
Models incorporating clinical and pathologic data
Several prognostic models have been developed that predict the risk of progression after surgery for localized RCC using clinical and pathologic variables ( Table 1 ). These models, some of which have been externally validated, may prove to be useful for selecting patients for adjuvant therapy. Each model has limitations. For instance, Kattan and colleagues acknowledged that predicting 5-year recurrence-free survival limits the usefulness of their nomograms, because it does not capture the 15% to 19% rate of recurrence beyond 5 years. In addition, the 2001 Memorial Sloan-Kettering Cancer Center (MSKCC) nomogram included both clear cell and nonclear cell histology. The investigators thus chose to exclude nuclear grade from their nomogram because of controversy regarding the grading of nonclear cell tumors. Given the association between grade and outcome, the impact of excluding nuclear grade on the predictive capacity of the nomogram has been questioned. The 2005 MSKCC nomogram addresses this concern by limiting the model to clear cell RCC (ccRCC), which allowed nuclear grade to be included as a predictive variable.
| Author | Year | Institution | Type | Study Population (n) | Inclusion | Variables | Outcome | Timepoint (y) | Concordance Index | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNM | Histology | Nephrectomy | Years | Symptoms | Performance Status | Tumor Size | pT | pN | Grade | Histology | Necrosis | Vascular Invasion | ||||||||
| Kattan et al | 2001 | MSKCC | Nomogram | 601 | T1–3c, N0/x, M0 | Papillary, chromophobe, ccRCC | Partial, radical | 1989–1998 | ✓ | ✓ | ✓ (1997) | ✓ | Recurrence-free survival a | 5 | 0.74 | |||||
| Zisman | 2002 | UCLA | Algorithm (low, moderate, high risk) | 468 | T1–4, N0, M0 | Any | Partial, radical | 1989–2000 | ✓ | ✓ (1997) | ✓ | ✓ | Overall survival, disease-specific survival, local recurrence-free survival, and systemic recurrence-free survival | 1, 2, 3, 4, 5 | NA | |||||
| Leibovich et al | 2003 | Mayo | Algorithm (score between 0 and 11) | 1671 | T1–4, Nx –N2, M0 | ccRCC | Radical | 1970–2000 | ✓ | ✓ (2002) | ✓ | ✓ | NA | ✓ | Metastasis-free survival | 1, 3, 5, 7, 10 | 0.82 | |||
| Sorbellini et al | 2005 | MSKCC | Nomogram | 701 | T1–3c, N0/x, M0 | ccRCC | Partial, radical | 1989–2002 | ✓ | ✓ | ✓ (2002) | ✓ | NA | ✓ | ✓ | Recurrence-free survival a | 5 | 0.82 | ||
The modified University of California at Los Angeles (UCLA) Integrated Staging System (UISS) has been externally validated. Like the MSKCC nomograms, the UISS timeframe is limited to 5 years. A limitation of the UISS is that it assigns patients to low-risk, medium-risk, or high-risk groups rather than predicting risk for an individual patient. A range of outcomes is expected within each risk category. The discriminating abilities of the 2001 MSKCC nomogram and the UISS were compared using a multicenter European cohort of 2404 patients. The concordance indices were 0.71 and 0.68 for the MSKCC and UISS models, respectively. The MSKCC nomogram improved discrimination of the UISS intermediate-risk category.
Additional predictive models exist that are based solely on preoperative variables such as gender, symptoms, and imaging findings, including necrosis, lymphadenopathy, and tumor size. The postoperative models, which include pathologic variables, discriminate better than preoperative models and are therefore more appropriate for selection of candidates for adjuvant therapy. Nonetheless, preoperative-only models may help select intervention versus active surveillance, and may prove useful for identifying patients for neoadjuvant therapy.
Models integrating molecular markers with clinical and pathologic data
Future efforts to determine risk of recurrence after nephrectomy may incorporate molecular markers. For example, addition of data regarding expression of 3 molecular markers (carbonic anhydrase IX [CA IX], vimentin, and p53) to clinical markers (metastasis, T stage, performance status) yielded modestly improved accuracy in predicting disease-specific survival in localized and metastatic ccRCC compared with the UISS (concordance indices 0.79 vs 0.75). More recently, the same group published a nomogram combining clinical, pathologic, and molecular data to predict disease-free survival after nephrectomy for localized ccRCC ( Fig. 1 ). The nomogram variables include expression of Ki-67, p53, endothelial vascular endothelial growth factor receptor 1 (VEGFR-1), epithelial VEGFR-1, and epithelial VEGF-D along with T stage and performance status. The predictive ability of the 5 molecular markers alone exceeded that of the UISS (concordance index 0.84 vs 0.78). The accuracy of the nomogram incorporating the clinical, pathologic, and molecular data was higher still (concordance index 0.90).
Similarly, the group from Mayo Clinic used immunohistochemistry to characterize expression of B7-H1, survivin, and Ki-67 in 634 patients treated with radical or partial nephrectomy for localized or metastatic ccRCC. The 3 molecular markers were each shown to be independently associated with RCC-specific death. Weighted scores were assigned to marker expression, which was dichotomized. The total score (range 0–7), termed BioScore, was able to discriminate cancer-specific survival ( Fig. 2 ). The investigators showed that the addition of BioScore improved the predictive ability of other models including TNM staging (concordance index 0.82 vs 0.79) and the UISS (0.82 vs 0.77).
Predictive models that include biomarkers are promising and may help select patients for adjuvant therapy. These models require independent validation and standardization of laboratory techniques before incorporation into clinical practice. Moreover, the costs of using biomarkers must be considered in context of the thus far modest improvement over the user-friendly, readily available clinicopathologic models.
Reported Adjuvant Trials
Radiotherapy
Initial adjuvant studies focused on improving local control with radiotherapy. Radical nephrectomy provides excellent local cancer control and recurrence of RCC is typically distant from the primary. Because local failure is uncommon, little role is expected for adjuvant radiotherapy. The data support this expectation.
In a prospective trial conducted from 1961 to 1970 at Newcastle General Hospital (Newcastle Upon Tyne, UK), patients with a completely resected primary tumor and no evidence of metastatic disease were randomized to observation (n = 49) or adjuvant radiation to the renal bed, incision, and para-aortic nodes (n = 51). There was no significant difference in local recurrence, development of metastases, or survival. Significant side effects, including 4 deaths from liver failure, were attributed to the radiation.
In 1987, Kjaer and Frederiksen reported the results of a multicenter randomized trial in Copenhagen, Denmark. Between 1979 and 1984, patients with stage II and III RCC were randomized after nephrectomy to 50 Gy of external beam radiotherapy in 20 fractions to the kidney bed and nodes (n = 32) or observation (n = 33). Radiotherapy was associated with hepatic, gastric, and duodenal injury, but no improvement in relapse. In 19% of patients, radiotherapy complications contributed to the patient’s death.
Hormonal therapy
Some renal tumor cells express glucocorticoid receptors that can be blocked by hormonal agents, such as medroxyprogesterone acetate (MPA), which have reported activity in the metastatic setting. In 1987, Pizzocaro and colleagues reported a multicenter trial in which patients were randomized to 1 year of adjuvant MPA (n = 58) or observation (n = 62) after radical nephrectomy for nonmetastatic RCC. Sixty-two (51%) of the patients had at least T3 disease. After a median follow-up of 5 years, rates of relapse were similar in the intervention and control groups (32.7 vs 33.9%). Complications were common in the intervention group.
Immunotherapy
There is a strong rationale for immunotherapy in RCC. The essential role played by the host immune system is shown by case reports of spontaneous regression of metastatic disease after nephrectomy or ablation of the primary tumor, as well as the presence of tumor-infiltrating immune cells in nephrectomy specimens, which have shown antitumor activity. The primary tumor is believed to have an immunosuppressive effect that can be ameliorated by nephrectomy. The aim of immunotherapy is to augment the host immune response. Once the immune sink has been excised, it is posited that adjuvant immunotherapy can better treat the remaining subclinical disease that leads to recurrence. Various methodologies have been used, including administration of cytokines, vaccines, dendritic cell therapy, and allogeneic hematopoietic stem-cell transplant to take advantage of graft-versus-tumor effect.
An aspect of the antitumor immune response is believed to be mediated by CD8+ cytotoxic T lymphocytes and amplified by CD4+ helper T cells, which secrete cytokines including interleukin 2 (IL-2) and interferon α (IFN-α). Exogenous IL-2 and IFN-α have shown efficacy in metastatic disease, with response rates up to 20% and a 5% durable complete response for IL-2. Based on these findings, several randomized trials investigated IL-2 and IFN-α as adjuvant therapy but were unable to show a disease-free or overall survival benefit ( Table 2 ). In 1 trial, adjuvant chemoimmunotherapy was associated with worse 5-year overall survival when compared with control (58 vs 76%, P = .028).
| First Author | Year | Eligibility | Intervention | Control | N | Median Follow-up | Primary End Point | Outcome (Intervention vs Control) | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Pizzocaro et al | 2001 | Robson II or III | IFN-α | Observation | 247 | NA | 5-year OS | 66.5% vs 66.0% | .861 |
| Messing et al | 2003 | pT3–4a or N+ | IFN-α | Observation | 283 | 10.4 years | Median OS | 5.1 vs 7.4 years | .09 |
| Clark et al | 2003 | pT3b–4 or N+ or M1 (resected) | High-dose IL-2 | Observation | 69 | 22 months | 2-year DFS | 48% vs 55% | .431 |
| Atzpodien et al | 2005 | pT3b–4 or N+ or M1 (resected) | IFN-α + IL-2 + 5-fluorouracil | Observation | 203 | 4.3 years | 5-year OS | 58% vs 76% | .028 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






