Fig. 9.1
Anatomy of the right gastroepiploic artery
The gastric fundus is the region most distant from its arterial inflow and venous drainage and thus particularly susceptible to ischemia. Blood flow to the fundus was initially thought to rely on the RGEA communicating directly with the LGEA. However, studies on this topic differ and have suggested that a direct RGEA anastomosis with the LGEA only occurs approximately 23–70 % of the time [1–5]. In a cadaver study by Liebermann-Meffert et al., the authors found that the RGEA contributed approximately 60 % of the total blood supply to the gastric tube with the remaining portion distributed among collaterals from the LGEA (20 %) as well as a smaller, submucosal network of collaterals (20 %). Of note, they also reported that direct communication between the RGEA and LGEA is minute and that while the right gastric artery is often preserved in esophagectomy, its contribution to the vascularity of the gastric tube is negligible [6]. These findings underscore the importance of the RGEA in the success of the gastric conduit during esophagectomy.
Vascular Considerations in Esophagectomy
Studies have demonstrated that use of a gastric conduit, as opposed to jejunal or colonic, for esophageal replacement following esophagectomy is associated with similar, if not lower, rates of ischemia. However, this highly morbid complication still occurs with use of stomach, with estimates ranging from 0.5% to 10.4% of cases [7–9]. Research has shown that mobilization of the gastric fundus during esophagectomy is associated with a greater than 50 % decrease in gastric tissue oxygen tension and that this resulting degree of oxygenation is correlated with subsequent success of the esophagogastric anastomosis [10, 11]. Consequently, while some loss of tissue perfusion and oxygenation is unavoidable during this surgery, optimizing conditions for blood flow is critical for a successful anastomosis and good postoperative outcomes. These studies highlight the importance of careful, gentle manipulation and handling of the whole gastric conduit throughout the entirety of the operation to minimize local trauma, vascular torsion/kinking, or conduit tension or compression. From a physiologic perspective, it is also important to avoid worsening perioperative splanchnic hypoperfusion by minimizing the use of vasopressors and alpha agonists. Communication with the anesthesia team intraoperatively and critical care team postoperatively regarding the significance of avoiding these medications is key to maximizing oxygen tension in the newly mobilized gastric conduit.
Preoperative Evaluation of the RGEA
A detailed past medical and surgical history is critically important prior to esophagectomy. Known aorto-iliac occlusive disease or peripheral vascular disease, as well as any prior vascular intervention whether transabdominal or catheter-based, should raise concern for adequacy of gastric conduit perfusion after mobilization. An associated history of diabetes, given its known impact on both macro and microvascular disease, may also warrant a more focused evaluation. A dedicated computed tomography (CT) scan of the chest, abdomen, and pelvis is often part of the preoperative evaluation of the esophageal cancer patient. In addition to reviewing the tumor and nodal morphology and ruling out metastases, the surgeon should also evaluate the visceral aorta for extensive calcification. In the setting of the aforementioned comorbid conditions, evaluation of celiac and mesenteric arterial integrity may be achieved through modalities such as CT or magnetic resonance (MR) angiography or aortography.
Currently, patients who have a planned esophagectomy, do not routinely undergo any form of preoperative screening to ensure an appropriate diameter or size of the RGEA. Evidence from cardiac surgery has shown that preoperative evaluation of this vessel in the form of transabdominal ultrasound or multidetector CT is feasible and may be worthwhile in operative planning for coronary artery bypass revascularization [12, 13]. For example, in a study by Minakawa et al., the authors used preoperative sonography to evaluate the RGEA and identify patients with a threshold artery diameter of 2 mm for subsequent revascularization. All individuals that met this criterion preoperatively were found intraoperatively to have arteries sizeable enough for subsequent anastomosis. Furthermore, comparison of preoperative ultrasound measurements with postoperative angiography of this vessel was highly correlated and confirmed acceptability of this screening approach. Unfortunately, data regarding the use of preoperative evaluation of the RGEA in esophagectomy are lacking. However, in patients who may have a history of foregut surgery, previous exploratory laparotomy, prior cardiac surgery with an unknown graft, or aberrant or incomplete anatomic visualization on routine preoperative imaging, the use of either of these modalities with special attention to the RGEA may prove useful in operative planning.
Preparation and Mobilization of the Gastric Conduit
Given the infrequency with which dedicated imaging of the RGEA is obtained preoperatively, it is important that soon after entering the peritoneal cavity and establishing exposure that the RGEA is identified. There is tremendous known variability in the celiac and hepatic arterial system and thus the location and path of the RGEA [14]. While this vessel reliably originates just inferior to the pylorus and traverses along the greater curvature, its relationship with the LGEA is subject to change as described above [1]. As such, to avoid accidental injury, it is advantageous to locate and establish the RGEA’s anatomic relationship and path early upon entering the abdomen prior to proceeding further in the course of the operation. This is especially true during any abdominal reoperation as adhesions may distort or obscure the precise anatomy of the omentum, transverse colon, and greater curvature of the stomach.
During the preparation of the gastric conduit, the greater omentum is separated from the greater curvature of the stomach. At this point in the operation, the surgeon should be extremely mindful of the previously identified course of the RGEA. Accidental injury, or excessive manipulation, of this vessel during dissection of the omentum can cause irreparable vascular compromise and subsequently result in an inability to use the stomach as a conduit for esophageal replacement [15]. Consequently, it is recommended that a minimum of 2.0 cm clearance be given between the RGEA and the omentum to be divided to avoid accidental mechanical or thermal injury (Fig. 9.2 ) [16]. Additionally, particular attention should be given when the dissection approaches the pylorus as the RGEA courses deep and posterior to the duodenal bulb to its origin from the gastroduodenal artery. The gastrocolic ligament and omentum are often fused with the transverse mesocolon in this location. Careful separation of these planes is required to avoid traction injury to the RGEA or its accompanying veins. This dissection also promotes easier passage of the conduit cephalad while decreasing subsequent anastomotic tension.
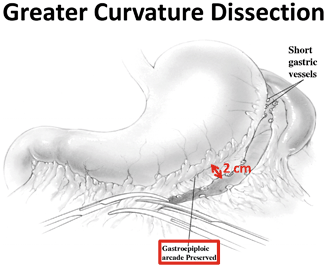

Fig. 9.2
Greater curvature dissection
Esophagectomy with the use of a gastric conduit involves a delicate balance of obtaining appropriate reach of the conduit while preserving vascularity to the esophagogastric anastomosis. Ensuring an appropriate length of conduit is key not only for achieving a tension-free anastomosis but also for minimizing reflux in the patient postoperatively [15]. Transferring of the conduit cephalad into the chest or neck is a critical step in the course of the operation. During this time, it is important to avoid excessive stretch, torqueing, or twisting that may result in stenosis, dissection, or a traction injury to the RGEA. Maintaining collinear movement of the conduit with its vascular pedicle will help to safeguard against inappropriate twisting or rotation during mobilization.
Additionally, after the tubularized stomach has been relocated to the chest, the surgeon should inspect the conduit to ensure that there is no excessive compression at the diaphragmatic hiatus. In recognition of this, we routinely open the hiatus anteriorly to the pericardial reflection, ligating the crossing phrenic veins. Omitting these safeguarding steps may result in significant vascular compromise to the conduit with identification after it is too late. Arterial compromise typically presents early postoperatively with acidosis and evidence of a systemic inflammatory response syndrome (SIRS) due to conduit necrosis. Venous compression is more insidious with full thickness necrosis often delayed until postoperative days 5–7.
Techniques for Improving Tissue Oxygenation
Tension-Free Anastomosis
Achieving appropriate length of the gastric conduit can often be an issue, especially for cervical anastomoses. The stretch placed on the stomach when attempting to reach the cervical esophagus may result in compromised blood flow, particularly in the relatively oxygen-deprived fundic region of the stomach. A generous Kocher maneuver and careful separation of the gastrocolic ligament from the transverse mesocolon aid in facilitating appropriate length.
Additionally, novel techniques have been described to address this issue of tension on the conduit and to allow for sufficient reach [17]. For example, noting the relative redundancy of the greater curvature in comparison with the strained lesser curvature, some authors have advocated for the use of a lengthening procedure termed “angleplasty.” In this technique, the point of tension at the angle of the lesser curvature is divided transversely through the seromuscular layer for a distance of 4 cm exposing the submucosa. This is followed by a longitudinal incision for approximately 4 cm through the gastric wall with subsequent closure of the incision using vertical seromuscular Lembert sutures [18]. By lengthening the gastric tube, this procedure may allow for a tension-free anastomosis and as a result improved arterial flow and reduced venous congestion in the proximal portion of the stomach.
Finally, as long as a cancer-free esophageal resection margin can be achieved, another simple technical maneuver to reduce tension when conduit length is limited is to change the level of the planned anastomosis from cervical to intrathoracic. Multiple studies have consistently demonstrated lower anastomotic leak rates for intrathoracic reconstructions [19, 20]. Thus, although an unanticipated change in the operative plan is not ideal, it is often preferable to a dubious anastomosis.
During any esophagectomy, but particularly in the context of a tenuous RGEA, creating a tension-free anastomosis is critical to a successful patient outcome. Use of a generous Kocher maneuver, “angleplasty,” and alterations to the anastomotic level are techniques the surgeon may employ to mitigate this concern as best as possible.
“Supercharging”
The territory most vulnerable to ischemia in the gastric conduit is the proximal portion of the stomach in the area of the fundus. This is primarily attributed to the unfortunate fact that after mobilization and transposition of the stomach into the chest or cervical region, this portion of the conduit is farthest away from its nutrient arterial inflow and venous drainage. This area is also where the esophagogastric anastomosis occurs. Thus, a potentially ill-fated situation occurs, whereby the area most susceptible to ischemia is also the region most in need of a robust blood supply for healing.
With this in mind, a technique that has received considerable attention for patients requiring esophagectomy is “supercharging.” The use of this method was first reported in 1947 and has increasingly been reported in the literature [15, 21]. “Supercharging” involves creating additional microvascular anastomoses to increase blood flow to the gastric conduit or, in some cases, the pedicled jejunal or colonic substitutes. While some surgeons may routinely use this procedure, more often it is selectively implemented to augment blood flow. In this context, the use of “supercharging” may prove invaluable as a salvage technique, particularly in the case of a tenuous conduit or compromised RGEA. The value of this procedure lies in its potential to not only increase arterial flow but also enhance venous drainage from the conduit. The latter is often a concern following esophagectomy, in particular when there is marked gastric distention at either the thoracic inlet or the diaphragmatic hiatus, or when the conduit is on tension or has a particularly long cephalad reach.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





