Chapter 3.12
Inflammatory bowel disease pathogenesis
Lynnette R. Ferguson1,2 and Liljana Gentschew3
1University of Auckland, Auckland, New Zealand
2 Nutrigenomics, Auckland, New Zealand
3 University of Kiel, Kiel, Germany
Inflammatory bowel disease (IBD) is the generic name for a group of chronic, relapsing, debilitating disorders of the small or large intestine or both. They typically develop in the teenage years, resulting in adverse symptoms, including abdominal pain and cramping, diarrhoea, rectal bleeding and malabsorption. The two main forms of IBD are Crohn’s disease and ulcerative colitis (UC), differing in location and severity. Once the disease begins, Crohn’s disease and UC tend to fluctuate between periods of inactivity (remission) and activity (relapse). Crohn’s disease can affect any part of the GI tract. However, it predominantly involves the terminal ileum and the beginning of the colon, whereas UC is limited to the rectum and colon. In Crohn’s disease, all layers of the intestine are involved. In contrast, UC affects only the superficial layers of the colon. It is believed that the clinicopathological diversity in UC and Crohn’s disease may be a reflection of distinct immune-genetic pathways [1,2].
The prevalence and incidence of IBD have been increasing worldwide over the last decades, particularly in industrialised countries [3]. Northern Europe, the UK, North America and New Zealand have shown the highest incidence and prevalence of Crohn’s disease [4]. However, these values have also grown in other countries of the world, i.e. southern and central Europe, Asia, Africa and South America [5]. The suggestion is that urbanisation and industrialisation, especially environmental factors such as diet, may be responsible for the changes in incidence [6].
Both UC and Crohn’s disease have a multifactorial aetiology, with a genetically determined susceptibility [2,7] that is only revealed in the presence of environmental factors such as adverse diet. While dietary factors may act directly on the GI tract, they also appear to act on the GI microbiota, leading to effects on processes that are essential for GI metabolism [8,9]. The nature of the genetics of IBD gives some clues as to disease aetiology, with 163 genes identified thus far [2]. Genetics relates strongly to disease characteristics. A general scheme summarising the interplay of such factors appears in Figure 3.12.1.
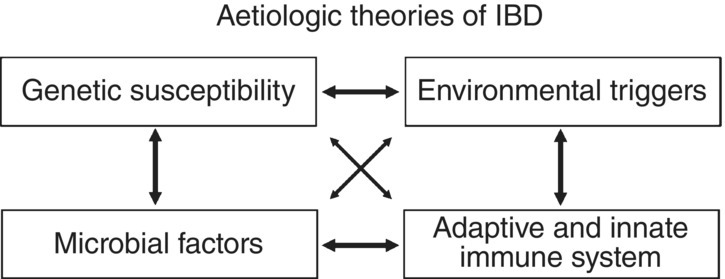
Figure 3.12.1 Interaction of various factors that are known to lead to chronic intestinal inflammation.
3.12.1 Underlying mechanisms of immune dysregulation in inflammatory bowel disease
Several studies implicate that the mucosal immune system and the intestinal epithelium are major factors in the pathogenesis of IBD [1,10,11]. In this context, animal models and human studies including genome-wide association studies (GWAS) have provided important insights into the immune-pathogenesis of IBD [2]. Various components of the mucosal immune system appear to be involved, including luminal antigens, intestinal epithelial cells, cells of the innate and adaptive immune system, and their secreted mediators [12,13]. Thereby, the integrity of barrier organs is maintained by the interplay between epithelial cells, or mucus layer, and the innate and adaptive immune systems [12,14].
Crohn’s disease is characterised by abnormal intestinal permeability, defects in mucus production and an inadequate, progressive production of proinflammatory cytokines such as tumour necrosis factor (TNF) alpha, interferon (IFN) gamma and interleukin (IL)-17 that induce intestinal inflammation [12]. Aberrant secretion of several cytokines by epithelial cells may initiate and perpetuate intestinal inflammation. The primary mediators of inflammation in Crohn’s disease are the Th1 cytokines IL-12, IFN-gamma and TNF [15,16]. Concerning this, lymphocytes, cytokines and adhesion molecules are dysregulated, resulting in a primary failure of regulatory lymphocytes and cytokines, such as IL-10 and transforming growth factor (TGF) beta. Various other factors have been implicated in the pathogenesis of Crohn’s disease, but their mechanism of action is often unknown [15,17,18].
3.12.2 Genetic factors in the development of inflammatory bowel disease
Epidemiological and family studies have provided convincing evidence that genetic factors play an important role in IBD [4,7,19,20]. Compared to UC, Crohn’s disease tends to be more common among relatives of patients with Crohn’s disease, and family and twin studies support a stronger genetic influence in Crohn’s disease than in UC [21–23]. An increasing number of studies demonstrate that Crohn’s disease appears more often in first-degree relatives who are not geographically living together, or at the same time [24]. Twin studies have shown that monozygotic twins have a much higher rate of disease concordance than dizygotic twins [25]. Several family and twin studies indicate that different genetic abnormalities can be broadly characterised as causing defects in mucosal barrier function, immunoregulation or bacterial clearance. Genes that are linked to innate immunity (e.g. NOD2), autophagy (ATG16L1, IRGM, ATG5), defective barrier (including ECM1, CDH1, LAMB1, HNF4A and GNA12), IL-10 signalling (e.g. STAT3, IL10RB, IL22 and IL26) and adaptive immunity (e.g. IL23, IL23R, IL17) have been discovered as key loci in Crohn’s disease [2,26].
Genetic and genomics research are rapidly growing areas, and recent studies have lead to advances in understanding of the molecular mechanisms of Crohn’s disease. Genome-wide association studies have furthered our understanding of the genetic architecture of IBD by discovering genes and loci that confer susceptibility to Crohn’s disease. Susceptibility loci that are associated with Crohn’s disease attaining genome-wide significance (P < 5 × 10−8) and statistical power of GWAS are supported by a large sample size. To this point, 163 IBD susceptibility loci have been discovered [2].
There is considerable similarity between IBD and risk factors for other autoimmune diseases. The primary genes are involved in innate and adaptive immunity. Many IBD loci are also implicated in other immune-mediated disorders, most notably with ankylosing spondylitis and psoriasis. Also, there is considerable overlap between susceptibility loci for IBD and mycobacterial infection. The relationships among IBD and related disorders are illustrated in Figure 3.12.2.
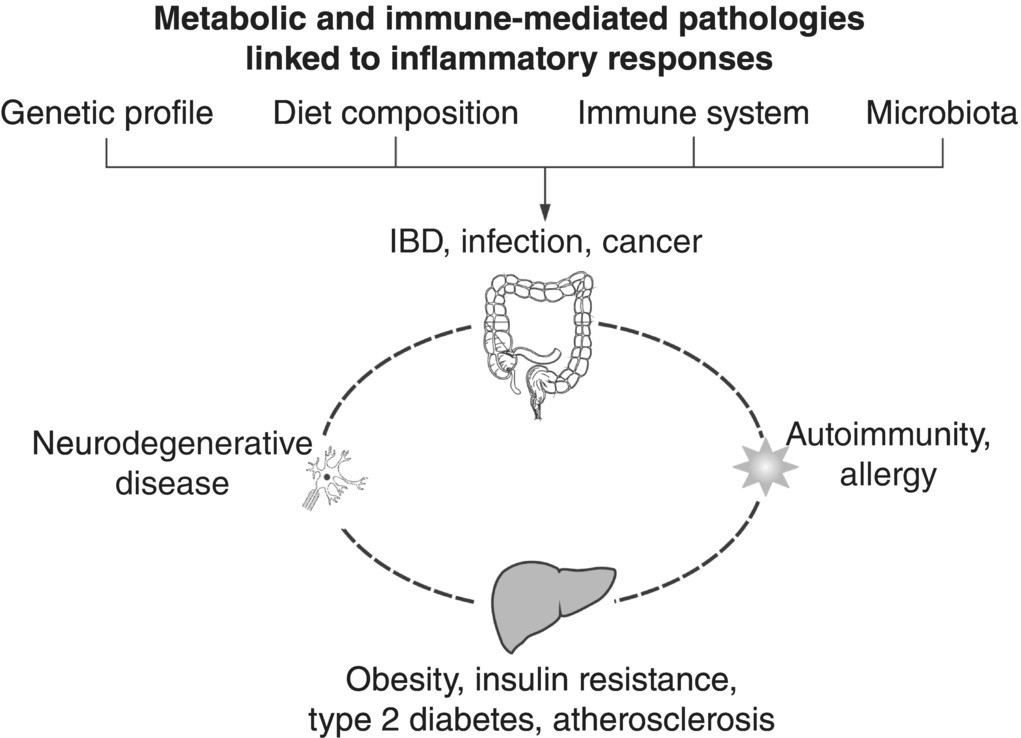
Figure 3.12.2 The link of inflammatory signalling response in various tissues to the development of obesity-related alterations, neurodegenerative disease and autoimmunity that are all influenced by host genetics, diet composition, immune system and microbiota. Modified from Renz et al. [78].
3.12.3 Dietary risk factors in inflammatory bowel disease
Diet is a major factor in both the aetiology and progression of the disease [1]. There are a limited number of high-quality studies that have unequivocally associated dietary intake with subsequent development of the disease. These take the form of excessive amounts of certain nutrients, deficiencies in others or excess energy intake with subsequent development of obesity. The literature is also sometimes confused between current diet and pre-illness diet of patients with Crohn’s disease. For example, Medline and the Cochrane Library were searched for clinical trials and meta-analyses in the scope of diet and nutrition in IBD [27]. These authors identified many studies in small cohorts of patients claiming that intake of Western-type diet constituents, including high saturated fat, refined sugar and low intake of fruits, vegetables and non-starch polysaccharides (NSP), affects the expression of IBD. Unfortunately, however, such studies are often compromised by insufficient data or methodological limitations, and do not provide unequivocal evidence to incriminate any particular dietary factor.
An example of a well-designed study is provided by Sakamoto and co-workers [28] in their Japanese populations. Cases were patients with IBD aged 15–34 years (111 UC and 128 Crohn’s disease) within 3 years after diagnosis in 13 hospitals. One control subject was recruited for each case, matched for sex, age and hospital. A semi-quantitative food frequency questionnaire (FFQ) was used to estimate pre-illness intakes of food groups and nutrients. A higher consumption of sweets was positively associated with UC risk and, more generally, the consumption of sugars, sweeteners and sweets was positively associated with Crohn’s disease risk. The intakes of total fats, monounsaturated fatty acids and polyunsaturated fatty acids (whether n-3 or n-6 PUFA) were positively associated with Crohn’s disease risk. With respect to micronutrients, the intake of vitamin C was negatively related to UC risk, while the intake of vitamin E was positively associated with Crohn’s disease risk. Although this study suffered from the shortcomings of recall bias, the findings reinforced the importance of dietary factors for IBD prevention.
Carbohydrates
Dietary carbohydrates can be divided into three main groups: sugars, in the form of monosaccharides and disaccharides, oligosaccharides such as maltodextrin, and polysaccharides including starches and NSP. The majority of research on the role of carbohydrates in IBD has focused on sugars and NSP.
A case–control study considered the intake of confectionery, preserves, biscuits and cakes 1–3 years prior to the onset of disease. The sample population was a group of 63 German patients with Crohn’s disease, using a validated postal questionnaire [29]. Intakes were significantly higher in the patients compared with those of 63 matched controls who recorded their current diet. Other workers [30–33] also studied pre-illness diet to show that patients with IBD consumed more sugar than age- and sex-matched population groups. Geerling et al. [34] studied pre-illness diet and found a significantly higher carbohydrate intake in patients with Crohn’s disease compared with controls and a tendency toward higher sugar intake. Sakamoto et al. [28] in Japan used a semi-quantitative FFQ to compare pre-illness diet in 108 patients with Crohn’s disease and 126 patients with UC with the diets of 211 controls. Increasing consumption of sugars, sweeteners and sweets was positively associated with increased risk of Crohn’s disease. Higher consumption of sweets was also positively associated with UC risk. In contrast to the other studies mentioned, this study adjusted for total energy intake. More generally, it seems that high intakes of mono- and disaccharides consistently increase the risk of developing either form of IBD.
High vegetable intake and increased fruit, possibly through increased NSP intake, appear to reduce the risk of both forms of the disease. Several case–control studies have more specifically investigated an association between NSP intake and IBD risk. Persson et al. [35] found that the relative risk of Crohn’s disease decreased with a high intake of NSP but this was defined as >15 g/day, a relatively low intake. In contrast, Thornton et al. [30,31] and Sakamoto et al. [28] found no difference between the NSP intake of patients with IBD and controls.
Fats and oils
The bulk (c. 95%) of edible fats and oils consist of triglycerides, whose structure is described as three fatty acids on a glycerol backbone. The predominant fatty acid will determine whether this is classified as a saturated, monounsaturated or polyunsaturated fat. The ratios of saturated and unsaturated fatty acids in the structure also determine physical characteristics, including melting point and stability. Technically, if a triglyceride is solid at room temperature, it is termed a fat, while if it is liquid at this temperature, it is termed an oil.
The most important natural sources of dietary fat are meats and dairy products. However, a major source of these in the current diet is provided by oils derived from vegetable sources, various spreads and associated products, including baked goods and confectionery products. Historically, the vegetable oil industry has relied heavily on hydrogenation in order to produce the types of stable fats used for frying, baking and table spreads, including margarines. In parallel with this, major human dietary sources have moved away from predominantly animal sources such as butter, ghee, tallow and lard.
The introduction of margarine in Europe coincided with the first reports of Crohn’s disease, and a causal relationship was proposed. The study by Sakamoto et al. found a significantly positive association between consumption of margarine and development of UC [28]. Sonnenberg linked data on margarine consumption obtained in five countries from 1962 to 1982 with mortality data for Crohn’s disease over the same period, but found no statistically significant association between them [36]. As mortality associated with Crohn’s disease is low and data on the incidence of the disease were not reported, the results should be viewed with caution.
A case–control study design was used to study pre-illness changes in Italian diet as a risk factor for IBD [37]. The study considered 83 new cases of IBD (41 UC, 42 Crohn’s disease) in comparison with 160 healthy controls. A validated questionnaire was used to record portions per week of 34 foods and beverages, before onset of symptoms was recorded. The study also recorded duration of symptoms before IBD diagnosis, presence of specific symptoms and their impact on subjective changes in usual dietary habits. In patients with IBD who did not change dietary habits, moderate and high consumption of margarine was associated with increased risk of UC, while high consumption of red meat and cheese was associated with increased risk of Crohn’s disease. The authors concluded that more than one-third of patients with IBD changed their dietary habits before diagnosis. However, high intakes of margarine, red meat and cheese increased the risk of both forms of IBD in this population group. These are good sources of both saturated and trans fats.
The association between the incidence of Crohn’s disease and dietary changes in Japan between 1966 and 1985 was examined by Shoda et al. [38]. An increased incidence of Crohn’s disease was strongly correlated with increased intake of total fat, animal fat and n-6 polyunsaturated fatty acids, and a relatively decreased intake of n-3 fatty acids. There is evidence that n-3 polyunsaturated fatty acids (PUFAs), particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), antagonise the production of inflammatory eicosanoid mediators from arachidonic acid, suppress production of some inflammatory cytokines and downregulate the expression of a number of genes involved in inflammation [39,40]
Dietary linoleic acid is an n-6 polyunsaturated fatty acid, which is metabolised to arachidonic acid, a component of colonocyte membranes. Metabolites of arachidonic acid have proinflammatory properties and are increased in the mucosa of patients with UC. In 2009, the IBD in EPIC study investigators considered the intake of linoleic acid as a factor in the aetiology of UC [41]. They utilised a nested case–control study within the EPIC European prospective cohort study. The data showed that a high dietary intake of linoleic acid, as assessed from food frequency questionnaires, significantly increased the risk of developing UC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






