Chapter 3.13
Inflammatory bowel disease nutritional consequences
Konstantinos Gerasimidis
University of Glasgow, Glasgow, UK
The aetiology of malnutrition in inflammatory bowel disease (IBD) is multifactorial and may present as protein energy malnutrition (PEM), altered body composition, micronutrient deficiencies and poor bone health. In children, growth failure and pubertal development delay can be additional outcomes of poor nutritional status which further complicate disease management. Reduced dietary intake, altered energy/nutrient metabolism, increased GI nutrient losses and drug–nutrient interactions are all implicated in the origins of malnutrition in IBD [1].
3.13.1 Protein energy malnutrition
Protein energy malnutrition is common at the time of diagnosis and the patient’s nutritional status fluctuates during the disease course [2]. History of weight loss, underweight and thinness (defined as a low Body Mass Index (BMI)) are common presenting features of the newly diagnosed patient and frequently accompany episodes of disease relapse [3]. Protein energy malnutrition is more common in Crohn’s disease compared with ulcerative colitis (UC) and is seen in approximately 60% and 35% of newly diagnosed patients respectively [3]. Apart from the higher prevalence of PEM in Crohn’s disease, there is no consistent evidence to link it with other specific disease characteristics (e.g. disease location, diagnosis delay). However, recent data suggest that fewer patients are now seen with PEM compared with previous studies, and a large proportion of patients are overweight or obese at diagnosis, particularly in UC.
In a large North American study of 783 newly diagnosed IBD children, low BMI was seen in 22–24% with Crohn’s disease and 7–9% with UC. In contrast, 10% and 20–30% of children with Crohn’s disease and UC respectively had a high BMI consistent with being overweight or obese [4]. The obesity epidemic in the general population, combined with earlier disease recognition of IBD nowadays, may explain these secular changes in patterns. There is limited evidence on the progression of undernutrition after diagnosis. In the only study undertaken thus far, a similar proportion of children with Crohn’s disease had short stature (height z-score ≤ −2) and 50% fewer children were classified as thin (BMI z-score ≤ −2 SD) at follow-up compared with disease diagnosis. Growth and nutritional retardation at diagnosis, young age, male gender and extraintestinal manifestations at diagnosis were predictors of poor prognosis at follow-up [5].
3.13.2 Body composition
There are several reasons to speculate why body composition in patients with IBD may differ from that of healthy people. Secretion of proinflammatory cytokines may alter energy metabolism, protein turnover and energy substrate utilisation, whereas the use of corticosteroids increases body fat with catabolic effects on lean mass. Physical activity, on the other hand, was reported to be low in adult patients with IBD and correlated inversely with fat mass (FM) [6].
There are few studies that have assessed body composition in IBD. Lean body mass or fat-free mass (FFM) has been consistently reported as significantly lower than healthy control groups whereas occasionally gender-specific associations with FM have been found [7]. Thayu et al., in a well-designed prospective study of newly diagnosed children with Crohn’s disease, also reported gender-associated differences with body composition [7]. Fat mass and lean mass for height (adjusted for age, race and pubertal stage) were lower in female than in male patients. Compared with a cohort of healthy controls, body composition in females was more consistent with wasting (low lean and FM) whereas in males there was mostly preservation of FM and deficits in lean mass consistent with cachexia. No consistent associations have been observed between body composition, clinical activity, disease location or diagnosis delay. Interestingly, normalisation of BMI at 2 years follow-up has not been associated with a significant increment in FFM in children with Crohn’s disease [8], which implies that changes in body weight or BMI for age are not good proxies for body composition changes in IBD so simple bedside techniques of body composition assessment, for routine use in clinical practice, are required.
Nevertheless, interpretation of body composition data in disease has to be approached with caution since the underlying assumptions about the composition of body compartments may be invalid [9]. Most in vivo body composition methods used in previous IBD studies, e.g. dual energy X-ray absorptiometry (DEXA), have been tested and validated in healthy individuals or animal cadavers and their applicability in chronic illness is questionable given the changes that may occur in the hydration level and distribution of fluids within the body compartments [9]. Assessment of the validity of these techniques in an IBD population and replication of these results with the application of more sophisticated methods need to be explored. The use of functional tests (e.g. handgrip strength) has been proposed as a proxy estimate of FFM in patients with IBD but these techniques lack specificity. Wiroth and colleagues found that patients with Crohn’s disease in clinical remission have overall lower muscle performance than healthy controls, but this was independent of FFM levels [10].
3.13.3 Bone health
Bone mineralisation is an important aspect in the care of patients with IBD, particularly as peak bone mass, attained during adolescence, was found to be the most important determinant of lifelong skeletal health [11]. Osteopenia and osteoporosis are important extraintestinal manifestations in IBD that may be related to increased risk of fractures [11,12]. In adult studies, a 60–70% higher risk for vertebral and hip fractures incidence was found for patients with IBD compared with healthy controls [12,13] but there is no strong evidence to suggest that in IBD children, bones are more brittle and that they experience more fractures compared with their healthy peers. It is difficult to interpret these discrepancies between adult and paediatric studies but it can mean that children with IBD may be more predisposed to have brittle bones that are at higher risk of fracture in adulthood and may occur earlier than in healthy adults (e.g. before menopause). The use of oral steroids to induce disease remission in adults might explain the higher risk of bone fractures; children are more likely to be treated with enteral nutrition (EN) rather than oral steroids to induce remission. Moreover, it is also possible that vertebral fractures occur in IBD children but these may be asymptomatic and hence remain undiagnosed [14].
A disease-associated effect is well documented, with poor bone health seen more often in Crohn’s disease than UC. Disease location, duration and history of disease activity were risk factors in some but not all studies [12,15–18]. Recent data suggest that afflicted children have the potential to improve their bone mineral density by the time they reach early adulthood [19].
Burnham et al. [20] reported that the difference in bone mineral content between Crohn’s disease and healthy controls was eliminated when they used a regression model to account for differences in lean mass while Sylvester et al. showed that changes in bone mineral content during a period of 2 years post diagnosis were positively associated with concomitant increments in FFM [8]. These findings suggest that decreased mechanical stress may be an important factor for reduced bone health in Crohn’s disease and this opens a treatment opportunity to improve bone mass by optimising lean tissue gain through nutritional support and weight-bearing exercise in patients with IBD [21].
Bone mineralisation in IBD can be negatively affected by undernutrition, low vitamin D status, the effect of proinflammatory cytokines on bone formation, resorption and osteoblast maturation [22] and the long-term use of high steroid doses [23,24]. As delayed skeletal maturation and sexual maturation are commonly seen in IBD, particularly Crohn’s disease, it is important to express the results not as z-scores for chronological age but accounting for pubertal staging and bone age [25].
3.13.4 Linear growth and short stature
Short stature and faltering linear growth are commonly encountered in IBD, and frequently precede disease diagnosis. Approximately 23–25% of paediatric patients have presented with deviation from their growth velocity and height for age centiles, or as significantly shorter than their healthy peers [26], and a proportion will fail to attain their genetic potential for linear growth, when their height deficits are compared with their estimated midparental target height [26]. The exact mechanisms by which growth impairment occurs in IBD are unclear but it is believed to be an interplay between undernutrition, delayed puberty, the effect of circulating proinflammatory cytokines and long-term use of steroids [27].
3.13.5 Delayed puberty
Delayed puberty is a frequent feature of young patients, more often in Crohn’s disease than UC, and in males than in females [17,23,28]. Mean delays in puberty of 0.7 and 1.5 years were found in Dutch and USA studies, respectively [23]. Delayed pubertal onset may influence linear growth and final adult height and could affect quality of life and self-esteem but the latter aspect has not been addressed prospectively.
Undernutrition has always been thought to be the main reason for delayed puberty in patients with IBD. However, puberty may be delayed despite a normal nutritional status. Observations in animal models of experimental colitis suggested that inflammation may have a direct adverse influence, independent of undernutrition, on the onset and progression of puberty (Figure 3.13.1) but relevant studies in patients with IBD are lacking. In vitro studies suggested that proinflammatory cytokines (e.g. tumour necrosis factor (TNF) alpha, interleukin (IL) 1beta, IL-6) can affect sex steroid production at the level of testes and ovaries [29].
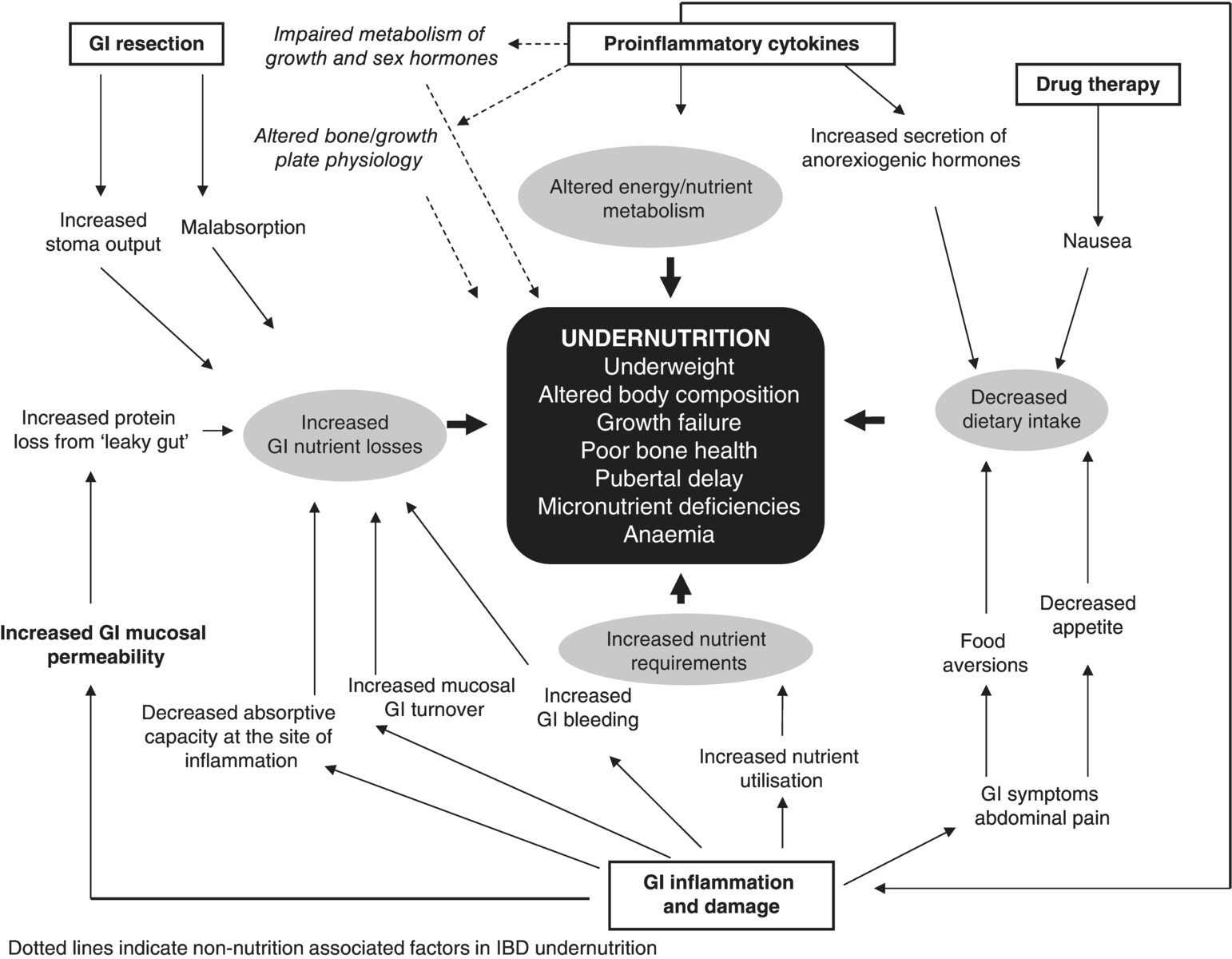
Figure 3.13.1 The aetiology and presentation of malnutrition in IBD. GI, gastrointestinal; IBD, inflammatory bowel disease.
3.13.6 Micronutrient status
Although clinical presentation of frank micronutrient deficiencies in IBD is very rare and largely limited to case reports, suboptimal circulating concentrations for virtually every vitamin, mineral and trace element have been reported previously, primarily in adult patients but also evident in the paediatric studies (Table 3.13.1). Antioxidant trace elements (e.g. Zn, Se, Cu) and vitamins (e.g. vitamins A, E, C, carotenoids) were the main nutrients consistently reported at suboptimal circulating concentrations in patients with IBD compared with healthy controls or the normal reference range (see Table 3.13.1). Serum vitamin D has been reported to be low in adult [30] and paediatric studies [31] and is an independent risk factor for poor bone health.
Suboptimal dietary intake, increased utilisation, malabsorption and increased enteric losses have all been postulated as causes of these nutritional deficiencies (see Figure 3.13.1). Some studies have linked nutritional deficiencies with clinical disease activity and inflammatory markers (see Table 3.13.1) but whether micronutrient depletion plays an important role in the pathogenesis and perpetuation of the mucosal lesions or is the result of these needs remains unknown. However, it must be remembered that changes in the plasma concentrations of many micronutrients and their association with systemic and clinical activity indexes can be an epiphenomenon of the acute phase response in inflammatory conditions like IBD [32]. Although reduced serum concentrations of micronutrients are often used to define deficiency states, these concentrations may better reflect disease activity and inflammation rather than being biomarkers of body tissue deficits [32]. A prime example is the transient decrease in plasma retinol binding protein and accordingly transported vitamin A plasma concentrations in the presence of the acute phase response in inflammatory conditions.
Table 3.13.1 Major circulating concentrations of multiple micronutrients in patients with inflammatory bowel disease
| Study | Participants | Assessed micronutrients | Outcomea |
| Hengstermann et al. 2008 | 167 IBD (132 in remission, 35 active); 45 HC | Vitamins: C, E, carotenoids Minerals : Se, Zn, Cu |
|
| Filippi et al. 2006 | 54 CD in remission | Vitamins: C, A, D, E, B1, B6, B12, E, folate, niacin, beta-carotene Minerals: Fe, Cu, Ca, P, Mg, Zn |
|
| D’Odorico et al. 2001 | 46 UC; 37 CD; 386 HC | Vitamins: A, E, carotenoids |
|
| Wendland et al. 2001 | 37 CD; 37 HC | Vitamins: C, E, A, carotenoids Minerals : GSHPx, Se |
|
| Geerling et al. 2000 | 23 CD; 46 UC (newly diagnosed); 69 HC | Vitamins: A, E, C, beta-carotene, B1, B12, folate Minerals: Mg, Cu, Zn, Se, GSHPx |
|
| Geerling et al. 1998 | 32 CD in remission; 32 HC | Vitamins: A, E, C, beta-carotene, B1, B12, folate Minerals: Mg, Cu, Zn, Se, GSHPx |
|
| bOjuawo & Keith 2002 | 38 UC; 36 CD (newly diagnosed) IBD; 40 HC | Minerals: Zn, Se, Cu |
|
| bLevy et al. 2002 | 22 CD; 10 HC | Vitamins: retinol, beta-carotene, alpha-tocopherol, gamma-tocopherol |






