This article provides an overview of infectious and inflammatory conditions associated with male infertility. These conditions may affect several components of the male reproductive tract and therefore have the ability to potentially alter sperm function. The effect of these conditions on male fertility is poorly understood and often underestimated.
Key points
- •
Reproductive tract inflammation is common in men with infertility and may be due to infections or noninfectious causes such as smoking, environmental toxins, vasectomy reversals, and urethral surgery.
- •
Although the presence of elevated levels of semen leukocytes is the most commonly used method to identify inflammation in the male reproductive tract, this is an inaccurate marker for inflammation.
- •
The use of empiric therapies such as antibiotics, antiinflammatories, and antioxidants may reduce semen leukocyte levels and improve sperm parameters for some infertile men with pyospermia.
- •
Chlamydia trachomatis and Neisseria gonorrhoeae screening for men with infertility is warranted in regions with a high prevalence of infection with these organisms.
- •
Viral infections, particularly human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) have been associated with male infertility.
Introduction
The accepted definition of infertility is the failure of a couple to achieve a successful pregnancy with 1 year of regular unprotected coitus. This condition is common, affecting almost 15% of couples worldwide, with a male factor implicated in up to 50% of cases. A variety of conditions cause male infertility, including congenital malformations, exposure to environmental toxins, genetic and endocrinological disorders, and infectious and inflammatory conditions (the last condition accounting for almost 15% of cases of male infertility). Inflammation is the body’s response to a noxious agent in an attempt to eliminate it; the inflammatory response includes vasodilation, increased blood flow, and leukocytic infiltration to the infected site. The male reproductive organs that may be susceptible to infectious or inflammatory insults include the prostate, testicles, and epididymis, and with spermatogenesis and adequate sperm function intimately related to the proper function of these organs, any state of infection or inflammation may potentially impair the function of these organs and lead to altered sperm function, production, or transit. This review discusses the infectious and inflammatory conditions that are associated with male infertility and describes the biologic processes involved, which lead to impairment in fertility and sperm parameters.
Introduction
The accepted definition of infertility is the failure of a couple to achieve a successful pregnancy with 1 year of regular unprotected coitus. This condition is common, affecting almost 15% of couples worldwide, with a male factor implicated in up to 50% of cases. A variety of conditions cause male infertility, including congenital malformations, exposure to environmental toxins, genetic and endocrinological disorders, and infectious and inflammatory conditions (the last condition accounting for almost 15% of cases of male infertility). Inflammation is the body’s response to a noxious agent in an attempt to eliminate it; the inflammatory response includes vasodilation, increased blood flow, and leukocytic infiltration to the infected site. The male reproductive organs that may be susceptible to infectious or inflammatory insults include the prostate, testicles, and epididymis, and with spermatogenesis and adequate sperm function intimately related to the proper function of these organs, any state of infection or inflammation may potentially impair the function of these organs and lead to altered sperm function, production, or transit. This review discusses the infectious and inflammatory conditions that are associated with male infertility and describes the biologic processes involved, which lead to impairment in fertility and sperm parameters.
Inflammation—effect on the male reproductive tract
Achieving normal fertilizing potential for the human male involves an intricate process of germ cell division and maturation, sperm transit through an elaborate maze of tubules, and the addition of fluids from accessory organs, allowing the germ cell to become fully functional. Secretions by the prostate gland, seminal vesicles, and bulbourethral glands, including essential lubricants and products like zinc, citric acid, α-glucosidase, and fructose are crucial to attaining final normal sperm physiology. The completion of this delicate process described involves an intricate interplay between various organs with patent ducts: all these processes are unfortunately susceptible to various inflammatory and infectious insults ( Fig. 1 ).
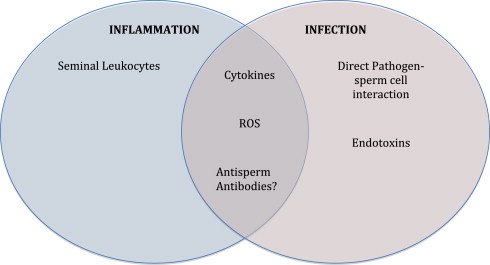
Inflammation is a complex process whereby the body reacts to infectious, traumatic, or chemical insults, causing an influx of activated leukocytes and various supporting cells and extracellular proteins. Although chronic inflammation usually develops after an acute symptomatic insult, it may also occur in tissue without a known history of injury or insult. In fact, most men who have a genitourinary (GU) tract inflammation have no symptoms of this inflammation.
It is this latter insidious process that is most worrisome in the male reproductive tract. Evaluation of testicular tissue specimens from asymptomatic infertile men reveals leukocytic infiltration in greater than 50% of men.
The impact of any inflammation or infection of the male reproductive tract on fertility depends on many factors, including the chronic versus acute nature of the disease and the type of invading pathogen ( Fig. 2 ). However, noninfectious inflammatory reactions may also affect the male reproductive tract. Lymphocytic infiltrates are commonly observed in patients with testicular seminoma, others with carcinoma in situ, and even in the contralateral testis of patients with unilateral tumors.
The inflammatory response is amplified by activated lymphocytes and macrophages through the release of cytokines, which includes a family of biologic response modifiers such as chemokines, interleukins, and growth factors. The main mediators of the inflammatory response in the male reproductive tract are the proinflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1α, and IL-1β. These signaling molecules are released by activated leukocytes and act in a synergistic fashion to allow cells to systematically eradicate the noxious insult. The cytokines IL-6, IL-8, and IL-10 are also released in states of inflammation and are found in varying levels in the semen of subfertile men with differing seminal defects, suggesting that semen cytokine profiling may potentially be used to detect inflammation in the male reproductive tract and may possibly help more accurately categorize subfertile men to aid in future treatment. Furthermore, IL-6 levels were also found to be elevated in patients with nonpathogenic inflammation, also suggesting its use as a marker of this condition.
Inflammation of the testicles may lead to spermatogenic arrest and a decrease in serum levels of testosterone and luteinizing hormone, thereby affecting the dual functions of spermatogenesis and steroidogenesis. The secretion of both TNF-α and IL-1α during the inflammatory response leads to an inhibition of steroidogenesis by Leydig cells. Furthermore, it seems that the effect of inflammation on spermatogenesis is cell specific, whereby spermatocytes and spermatids are affected, whereas spermatogonia are spared.
Evidence points to inflammation as a source of oxidative stress. Oxidative stress is an imbalance in the oxidant/antioxidant system, leading to an increase in reactive oxygen species (ROS), and is a significant cause of male infertility. In inflammatory states of the male reproductive tract, oxidative stress is generated predominantly by the infiltrating leukocytes and the proinflammatory cytokines that they generate. Phagocytosis by infiltrating phagocytes leads to accelerated oxygen consumption and generation of large amounts of ROS. Through activation of the xanthine oxidase system, cytokines similarly create a state of oxidative stress by generating high levels of ROS. These ROS react with polyunsaturated fatty acids found in abundance in the sperm cell membrane, leading to significant oxidative damage and a reduction in motility as well as fertilizing potential. Furthermore, spermatozoa have a small cytoplasm and hence a limited store of antioxidants, thereby limiting their ability to repair any structural damage caused.
Inflammatory and infectious conditions of the male reproductive tract may also lead to infertility due to damage of the reproductive tract organs affecting their function (altering the production or release of the secretions required to support sperm function) and due to scarring of the delicate ductal systems and subsequent anatomic obstruction. Nonregenerative epithelial cells lining the epididymis and testis are particularly vulnerable to scarring. The consequence of such scarring is stricture formation, which may occur in the ejaculatory ducts, thereby leading to a decreased ejaculatory volume and impaired fertility.
Inflammatory conditions potentially associated with male infertility
Prostatitis
The most widely used classification system for prostatitis is the National Institutes of Health (NIH) system, which includes 4 diagnostic categories of prostatitis. NIH category I prostatitis represents acute bacterial prostatitis; NIH category II prostatitis represents chronic bacterial prostatitis. Chronic nonbacterial prostatitis has been defined as NIH III prostatitis, being either NIH IIIA prostatitis (inflammatory) or NIH IIIB prostatitis (noninflammatory). Finally, NIH IV prostatitis represents asymptomatic inflammatory prostatitis, defined as the finding of significant pyospermia or elevated levels of leukocytes in the expressed prostatic secretions (EPS). Studying the potential impact of prostatitis on male fertility is important, as chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS: NIH III prostatitis) has been established as the most commonly diagnosed urologic problem in male patients younger than 50 years. In Canada, Nickel and colleagues reported prostatitis-like symptoms of voiding dysfunction, bladder spasms, and pelvic pain in 6% to 9% of men. The number of men with symptomatic CP/CPPS represents only a small fraction of the total group of men with prostatitis; this is true, as histologically proved inflammation may be present in more than 50% of men having prostate biopsies for benign conditions. Although it had been suggested that prostate inflammation can be diagnosed through the presence of elevated levels of leukocytes in EPS, in urine that is voided post-EPS, or in the semen, it is still not clear whether these parameters really are associated with prostate inflammation. For this reason, other diagnostic modalities such as cytokine profiling continue to be investigated as potential markers of prostatitis. IL-8, granulocyte elastase, and neopterin are 3 such biomarkers whose role in diagnosing prostate inflammation remains controversial.
Several studies have examined the effect of clinical prostatitis on sperm parameters; however, because of the use of different classification systems and the inherent problems in making a diagnosis of prostatitis, it remains difficult to reach a conclusion concerning the effect of prostatitis on sperm function. As early as 1971, Boström and Andersson examined sperm parameters in 31 patients with what would now be considered as NIH IIIA prostatitis and compared the results to 34 controls, with no significant difference found in sperm concentration, motility, or morphology. Similarly, in the largest study to date examining the effect of prostatitis on sperm parameters, Weidner and colleagues in 1991 examined 32 patients with a diagnosis compatible with NIH II prostatitis, 102 patients with NIH IIIA prostatitis, and 142 patients with NIH IIIB prostatitis and compared the results with those of a healthy control group of 42 patients, with no significant difference in sperm parameters detected. On the other hand, also in 1991, Christiansen and colleagues compared semen parameters in 50 patients with NIH IIIA prostatitis to those of 33 healthy controls and found a significant reduction in all 3 parameters of concentration, motility, and morphology in the prostatitis group. Similarly, Leib and colleagues compared semen parameters in 86 patients with NIH IIIA prostatitis with those in 101 healthy controls who were proved fertile and found a reduction in both sperm motility and morphology but not concentration. Krieger and colleagues also found an association between prostatitis and impaired sperm motility. In 2000, Pasqualotto and colleagues compared their patients with prostatitis with a control group and found a reduction in sperm motility only when associated with leukocytospermia. Menkveld and colleagues performed semen analyses on 34 patients with NIH IIIA prostatitis and 18 with NIH IIIB prostatitis, comparing the results with those of 17 healthy men, and detected a significant decrease in sperm morphology in the prostatitis group. Furthermore, Engeler and colleagues prospectively analyzed semen parameters of 30 patients with NIH IIIB prostatitis and compared the results with those of 30 healthy controls and found a significant reduction in sperm motility. In 2003, Ludwig and colleagues found no association between prostatic inflammation and standard semen parameters. On the other hand, Henkel and colleagues reported a significant reduction in sperm concentration and morphology in patients with NIH IIIA and IIIB prostatitis as compared with their control group but found significantly increased sperm motility ( Table 1 ). These contrasting results point to a possible but unconfirmed role for prostate inflammation in the alteration of all 3 semen parameters of concentration, motility, and morphology. These data suggest that men with symptomatic NIH III prostatitis CP/CPPS likely have impaired sperm parameters. On the other hand, there are no data to suggest that men with asymptomatic prostate inflammation have impaired sperm function. However, because of the inherent difficulty in proving that men with no symptoms of CP/CPPS may actually have prostate inflammation, in the absence of novel, noninvasive, and accurate markers of prostatic inflammation, it remains challenging to prove an association between asymptomatic prostate inflammation and infertility.
| Study/Year | Effect on Semen Parameters | ||
|---|---|---|---|
| Motility | Morphology | Count | |
| Boström et al, 1971 | 0 | 0 | 0 |
| Weidner et al, 1991 | 0 | 0 | 0 |
| Christiansen et al, 1991 | − | − | − |
| Leib et al, 1994 | − | − | 0 |
| Krieger et al, 1996 | − | 0 | 0 |
| Pasqualotto et al, 2000 | − | − | 0 |
| Menkveld et al, 2003 | 0 | − | 0 |
| Engeler et al, 2003 | − | 0 | 0 |
| Ludwig et al, 2003 | 0 | 0 | 0 |
| Henkel et al, 2006 | + | − | − |
Epididymitis
Epididymitis occurs less frequently than prostatitis and is responsible for about 1% of outpatient urology visits in North America, with most patients complaining of chronic symptoms lasting more than 3 months. Acute epididymitis usually occurs unilaterally and in up to 60% of patients, the testicle may be involved in a process termed epididymo-orchitis. The development of chronic disease is still unpredictable and difficult to diagnose. In men younger than 35 years, the pathogens implicated in epididymitis are usually sexually transmitted organisms, whereas in men older than 35 years, enteric pathogens are implicated. The detrimental effects of inflammation on the male reproductive tract are well documented; however, the sequelae of epididymal inflammation on male fertility remain poorly understood. In the epididymis, sperm is stored, sperm motility develops, and the sperm membrane matures to complete fertilizing potential. As a consequence, any inflammation of the epididymis may lead to a decrease in sperm count, altered motility, or sperm dysfunction. Scarring secondary to inflammation may finally lead to obstructive azospermia.
There is a paucity of data on the detrimental effects of epididymitis on sperm parameters. A review of the literature reveals only 5 studies investigating the impairment of sperm quality following a diagnosis of epididymitis, with the total number of patients from all studies of only 211 and the most recent study published more than 20 years ago in 1991. The consensus is that acute epididymitis usually leads to a temporary deterioration in sperm quality in most patients, with permanent damage including azospermia more than 2 years from diagnosis observed in some. Conversely, the incidence of either a history of or ongoing epididymitis in men with infertility is not known. However, the data available does suggest that epididymitis may have a detrimental effect on male fertility.
Orchitis
The true incidence of orchitis in the general male population remains unknown, with most of the knowledge extrapolated from data on epididymitis. Although an ascending canalicular bacterial infection may lead to orchitis, inflammation of the testis is usually associated with systemic viral infections, most notably mumps. Other viruses implicated in the development of orchitis include Coxsackie, Epstein-Barr, varicella, influenza, and HIV. Granulomatous orchitis is associated with tuberculosis, syphilis, and brucellosis. As mentioned earlier, there is a high prevalence of inflammatory changes in testicular biopsies from asymptomatic infertile men, leading to the thought that the effect of chronic testicular inflammation on male infertility is greatly underestimated. Furthermore, inflammatory changes in chronic orchitis resemble those typically seen in experimental autoimmune orchitis, allowing one to conclude that both these conditions may lead to a loss in the testicular immune privilege.
There is a scarcity of data on the impact of orchitis on sperm parameters. What complicates matters is the absence of valid serologic or seminal markers and the difficulty in noninvasively diagnosing orchitis without resorting to testicular biopsy. What is known is that despite adequate antimicrobial therapy and the absence of symptoms, ongoing inflammation occurs in men with a history of orchitis. It is clear that testicular inflammation is common in men with infertility. What is unknown is do men with infertility have a higher frequency and/or more extensive testicular inflammation than men with normal fertility and if the inflammation causes the infertility.
Urethritis
The urethra is considered a conduit through which the ejaculate containing mature sperm exits the body. Consequently, any inflammation of the urethra is unlikely to impair spermatogenesis or directly affect sperm quality or function. However, it is not completely implausible that an ascending infection progresses from urethritis to epididymitis and possibly to epididymo-orchitis, thereby leading to impaired fertility. Furthermore, urethritis can cause urethral scarring and stricture formation, leading to a decreased ejaculate volume and reduced fertility.
Pyospermia: a marker of reproductive tract inflammation?
Elevated levels of seminal leukocytes may be due to GU tract infections; however, there are multiple other reported causes for the elevated level of leukocytes, including certain exposures (smoking, environmental toxins, marijuana, and some medications), GU surgery such as vasectomy reversals and urethral surgery, and autoimmunity. Elevated levels of leukocytes may also just be a marker for apoptosis, as the leukocytes are recruited to recycle the dying sperm. One question that arises is whether elevated levels of semen leukocytes may act as a marker of infection in the GU tract. Multiple studies have now shown no correlation between elevated levels of semen leukocytes and bacteriospermia. In a large study on 7852 men, Domes and colleagues found no correlation between elevated levels of leukocytes and bacteriospermia, which mirrored the results reported by Wolff and colleagues and Yanushpolsky and colleagues.
Are the elevated semen leukocyte levels good markers of GU inflammation? Certainly, elevated leukocyte levels in the semen are associated with multiple alterations in men’s fertility potential, with poorer sperm parameters such as reduced sperm count, motility, and normal sperm morphology; reduced sperm function as measured by the sperm penetration assay (SPA); elevation of the sperm DNA fragmentation assays; and finally reduced pregnancy rates for couples with male-factor infertility. In small studies in which antibiotics were used to treat men with pyospermia, the leukocytospermia declined and the SPA results improved. Other groups have also successfully treated men with pyospermia using antioxidants and antiinflammatories, with reductions in the semen leukocyte levels and improved sperm parameters seen. These results suggest that elevated semen leukocyte levels are markers of GU inflammation for some men. However, not all men with GU inflammation have elevated levels of semen leukocytes.
However, until a standardized method to accurately and noninvasively diagnose GU inflammation is developed, one needs to continue to use an elevated semen leukocyte concentration as a marker of GU inflammation. Although there are only a few studies on the treatment of men with elevated semen leukocyte levels, the limited literature does suggest that the use of antibiotics, antiinflammatories, and/or antioxidants may lead to a reduction in the semen leukocyte concentrations and improved sperm parameters.
Infections—effect on the male reproductive tract
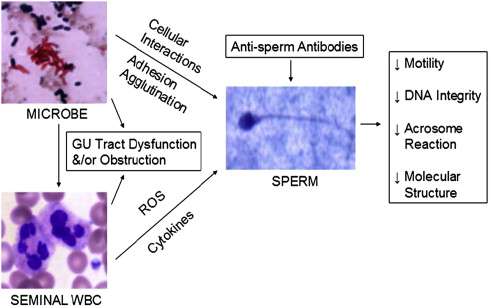
Infectious organisms could have a direct negative effect on sperm by directly interacting with sperm cells or potentially have a negative impact on fertility by a secondary effect either through the inflammatory process inherent in the infection or due to the release of toxins.
Any infection of the male genital tract leads to an inflammatory response whereby the body’s immune system attempts to fight off this infection. Chief among the causative infectious agents implicated in the male reproductive tract are gram-negative bacteria, which induce an inflammatory response due in part to the release of the endotoxin lipopolysaccharide present in the cell wall. Thus, one mechanism by which genital tract infections may lead to male infertility is by inducing a state of inflammation. Evidence suggests that invading leukocytes present during the inflammatory response enhance the detrimental effect of the invading pathogen on semen quality.
Infections may also impair fertility because of the direct effect of the pathogen on the sperm cell. Several bacterial and viral pathogens are known to interact with and cause damage to spermatozoa, including C trachomatis , Escherichia coli , Ureaplasma urealyticum , and HBV. E coli is known to adhere to spermatozoa and can cause sperm agglutination, whereas U urealyticum impairs sperm nuclear chromatin integrity. HBV decreases sperm motility by triggering a loss of mitochondrial membrane potential. These pathogens are also known to induce a state of oxidative stress by triggering the release of ROS, leading to further impairment of sperm function.
Infections— C trachomatis
C trachomatis infection has been recognized as one of the most common bacterial sexually transmitted diseases (STDs) in North America; therefore, assessing its impact on both male and female infertility is of utmost importance. One of the main initial obstacles remains the difficulty in diagnosing C trachomatis infection, with more than 50% of men and women not demonstrating any symptoms. Although it is acknowledged that C trachomatis leads to female infertility due to pelvic inflammatory disease (PID) and subsequent tubal obstruction, its impact on male infertility is still debatable, with studies revealing conflicting results. Despite being widely practiced, no clear guidelines exist for the screening of men with infertility for C trachomatis infection.
In men, C trachomatis infection may lead to urethritis, epididymitis, epididymo-orchitis, and even prostatitis and may impair male fertility by causing scarring and obstruction along the male reproductive tract. C trachomatis also leads to sperm cell apoptosis, as indicated by a high rate of DNA fragmentation in patients with C trachomatis infection. As with other STDs, coinfection with other infections is always a concern. Furthermore, unrecognized silent C trachomatis infection in male partners may easily be transmitted to their female counterparts, leading to the deleterious effects of this bacterium on female reproduction. When discussing the potential impact of C trachomatis infection on male fertility, it is important to evaluate the prevalence of this disease in the specific target population being examined ( Table 2 ). There is considerable variability in the reported incidence of C trachomatis infection in various populations, ranging in the literature from 0% to 42%. In the largest study to date comparing screening results for STDs between men with infertility and the general population, Domes and colleagues reported that the rate of C trachomatis infection was lower in Canadian men with infertility (0.3%) than in the general population. Therefore, although C trachomatis infection has a negative effect on female fertility and likely leads to impaired fertility in some men, it is important to establish the prevalence rates of the disease in the population of interest before considering launching any C trachomatis screening programs for men with infertility.
| Continent of Origin | Study/Year | Country | Number of Patients | Chlamydia Rate (%) | Effect on Semen Parameters | ||
|---|---|---|---|---|---|---|---|
| Motility | Morphology | Count | |||||
| Europe | Ochsendorf et al, 1999 | Germany | 125 | 1.6 | 0 | 0 | 0 |
| Pannekoek et al, 2003 | Netherlands | 153 | 4.6 | ne | ne | ne | |
| Eggert-Kruse et al, 2003 | Germany | 707 | 1.8 | 0 | 0 | 0 | |
| Hamdad-Daoudi et al, 2004 | France | 111 | 5.4 | ne | ne | ne | |
| Hosseinzadeh et al, 2004 | United Kingdom | 642 | 4.9 | 0 | 0 | 0 | |
| de Barbeyrac et al, 2006 | France | 277 | 1.2 | 0 | 0 | 0 | |
| North America | Bezold et al, 2007 | United States | 241 | 2.5 | − | − | − |
| Domes et al, 2012 | Canada | 5588 | 0.3 | ne | ne | ne | |
| South America | Vigil et al, 2002 | Chile | 284 | 38.6 | 0 | 0 | 0 |
| Africa | Gdoura et al, 2008 | Tunisia | 104 | 42.3 | 0 | 0 | 0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





