Infectious Diseases
Mechanisms of Bacterial Injury
Numerous factors predispose to microbial intestinal colonization and contribute to diarrhea, malnutrition, sepsis, and extraintestinal infections. Vigorous peristaltic forward propulsion of intestinal contents into the colon discourages bacterial colonization. This peristaltic defense is enhanced by the continuously secreted mucous layer that bathes the mucosal surface and mechanically prevents organisms from contacting enterocyte surfaces and adhering to them. Pancreatic enzymes in the intestinal lumen degrade bacterial toxins. Bacterial enteropathogens possess several distinct virulence properties (Table 6.12). The specific bacterial virulence pattern determines the way it interacts with the intestinal mucosa and influences the clinical syndromes that result.
TABLE 6.12 Mechanisms of Bacterial Pathogenicity in Small Intestinal Injury | |
|---|---|
|
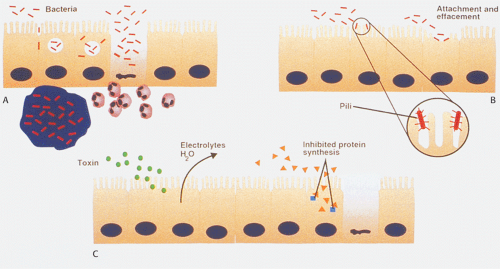
Since bacterial adherence to epithelial cells is an important prerequisite for intestinal colonization and virulence (Fig. 6.130), bacteria have developed several mechanisms to attach
to enterocytes (294). Enterocyte adherence results from specific interactions between a ligand expressed on the bacterial surface (sometimes called an adhesin) and a receptor on the epithelial cell surface (295). Various bacteria produce plasmid-encoded adhesive pili that radiate from the bacterial surface and recognize specific glycoconjugates on mucosal cells (296). Other bacteria attach to epithelial cells in such a way that the outer membrane of the enterocyte appears to wrap around it. This gives the impression that the bacterium is perched on an enterocyte pedestal. Bacterial toxins interact with receptors on the enterocytes surfaces and activate cellular signal transduction mechanisms, causing fluid and electrolyte secretion and toxigenic diarrhea.
to enterocytes (294). Enterocyte adherence results from specific interactions between a ligand expressed on the bacterial surface (sometimes called an adhesin) and a receptor on the epithelial cell surface (295). Various bacteria produce plasmid-encoded adhesive pili that radiate from the bacterial surface and recognize specific glycoconjugates on mucosal cells (296). Other bacteria attach to epithelial cells in such a way that the outer membrane of the enterocyte appears to wrap around it. This gives the impression that the bacterium is perched on an enterocyte pedestal. Bacterial toxins interact with receptors on the enterocytes surfaces and activate cellular signal transduction mechanisms, causing fluid and electrolyte secretion and toxigenic diarrhea.
Bacterial translocation implies the passage of viable bacteria from the gastrointestinal lumen through the mucosa to distant sites, such as mesenteric lymph nodes, spleen, liver, kidney, and blood (296). Mechanisms inducing bacterial translocation include disruption of the normal ecologic microbial balance with overgrowth of Gram-negative enteric bacilli, impaired host defenses, and physical disruption of the mucosal barrier. Some invasive bacteria cross the intestinal barrier via M cells (296). Others enter cells and remain trapped inside phagocytic vacuoles, where they utilize antiphagocytic strategies to continue to multiply and resist cellular defense mechanisms (297). Still others, such as Shigella, escape from phagocytic vacuoles to invade the cytoplasmic compartment. The pathogens then pass from cell to cell.
Epidemiologic Settings in which Infections Occur
Diarrheal diseases are a global problem, particularly affecting populations living in underdeveloped areas. Both children and immunologically naive travelers are susceptible to the enteric pathogens that heavily contaminate the water and food in places with poor sanitation. Food-borne and water-borne illnesses account for significant numbers of diarrheal outbreaks worldwide. The pathogenic bacteria must be ingested in large numbers to cause clinical disease. Food- and water-borne illnesses develop not only in countries with poor sanitation, but also on cruise ships, at picnics, and at fast-food restaurants. Diarrheal illnesses also lead the list of infectious diseases that affect the military. Travelers of all ages from industrialized countries experience high rates of diarrheal illness and other infections when they visit developing tropical areas (298). Regional differences in the consumption of unpasteurized milk or raw or undercooked fish, shellfish, or meat increase the risk of certain bacterial, parasitic, and viral infections. Mass production of eggs also plays a role in bacterial enterocolitis. Globalization of the food supply also increases the opportunity for widespread food contamination. Table 13.11 lists factors that should trigger suspicion of widespread food contamination. Diarrheal illnesses are also increasing in daycare centers, hospitals, and extended-care facilities. Nosocomial diarrhea is particularly prevalent in intensive care units and pediatric wards, and is an increasingly difficult problem for bedridden patients and their caregivers.
The synthesis of large numbers of antibiotics over the past several decades has resulted in increased bacterial antibiotic resistance (299). The emergence of drug-resistant bacterial strains has contributed significantly to the increase in nosocomial infections (300). Increased international travel offers new opportunities for outbreaks and plagues to develop, and offers opportunities for the genesis of new bacterial genetic variations (301). Finally, the increased frequency of immune deficiencies, whether from AIDS, immunosuppressive therapy, aging, or other alterations, places patients at risk for life-threatening infections.
Patterns of Gastrointestinal Infection
Most diarrheal illnesses are noninflammatory, usually arising in the upper small bowel from the action of an enterotoxin or other process that specifically alters the absorptive function of the villous tip. Patients with toxigenic bacterial infections present with dysenteric syndromes characterized by fever, abdominal pain, and numerous small-volume stools containing blood, mucus, and neutrophils. In contrast, patients with invasive bacterial infections usually have a colonic infection and diarrhea dominates the clinical picture. Neutrophilic mucosal infiltrates are the hallmark of acute invasive disease. Toxigenic organisms tend to produce less severe morphologic damage than invasive bacteria. Another type of enteric infection results in enteric fever, often with constipation early in its course. The organisms enter Peyer patches and regional lymph nodes and then become systemic infections, returning to the gut later. In order to establish the diagnosis of infectious diarrhea, the organism must be found in, or cultured from, the stool or intestinal tissues. Alternatively, one should be able to demonstrate a rise in specific serum antibodies to the organism. With the advent of recombinant DNA technology, genes for the heat-labile and heat-stable enterotoxins have been cloned, allowing diagnosis of the toxin-producing bacterial strains.
Bacterial Overgrowth Syndromes (Blind Loop Syndromes)
Bacterial overgrowth leads to malabsorption. Proposed mechanisms for the abnormal bacterial proliferation include (a) failure to clear bacteria from the upper GI tract, usually due to achlorhydria; (b) continuous seeding of the small bowel with colonic contents as a result of jejunal-colic fistulae or reflux following abnormalities of the ileocecal valve; and (c) motility disturbances. The diagnosis of bacterial overgrowth relies on three criteria: (a) presence of an increased intestinal volume, (b) demonstration of increased bacterial concentrations, and (c) a positive response to antibiotic therapy. Multiple conditions predispose to bacterial overgrowth (Table 6.13).
TABLE 6.13 Conditions Predisposing to Bacterial Infection and Overgrowths | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Most mucosal biopsies appear normal, but careful studies eventually demonstrate patchy mild histologic abnormalities that are easily missed by a single random biopsy or are so mildly abnormal as to be easily overlooked. In severe cases, one sees a spectrum of changes ranging from patchy, villous broadening and flattening to complete villous atrophy with crypt hyperplasia or hypoplasia. Numerous microorganisms, including bacteria and protozoa, adhere to the mucosa and are embedded in the unstirred mucous layer overlying the epithelium (Fig. 6.131). There is often an increase in the number of lamina propria mononuclear cells.
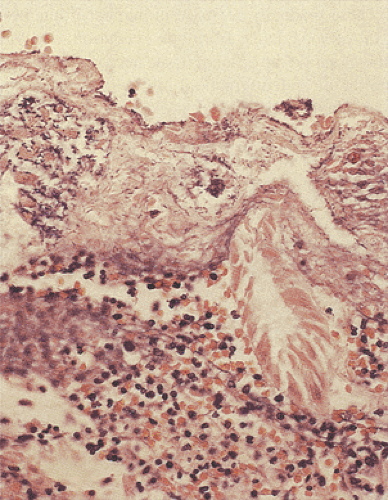 FIG. 6.131. Bacterial overgrowth. Note the presence of prominent collections of bacteria in the pyogenic membrane that was overlying an area of devitalized tissue. |
The recognition of specific pathogens may result from their localization in specific tissue sites, as in the colon (Table 13.12).
Specific Bacterial Infections
Escherichia coli Infections
E. coli bacteria are Gram-negative organisms that constitute part of the normal GI flora. They spread to contiguous structures when the mucosal barrier is damaged. The organisms tend to settle in necrotic tissues. Patients often have decreased host defenses as a result of underlying diseases. Several types of E. coli infections exist (Table 6.14) (302). However, they cannot be differentiated from one another on Gram stain or routine culture. The characterization of a strain of E. coli as an enteropathogen requires serotyping, tissue culture, immunochemical methods, or DNA hybridization studies, techniques that are not always routinely available.
Enteropathogenic E. coli (EPEC) account for outbreaks of severe fatal diarrhea in newborns (303), are a major bacterial cause of dehydrating infant diarrhea in developing areas, and cause traveler’s diarrhea. The organism is acquired by ingesting contaminated food or water. Risk factors for death include a young patient age and the virulence of the bacterial
strain. Almost all deaths occur before age 2. The organisms are not invasive; nor do they produce toxins. They colonize the proximal small intestine via specific attachment mechanisms, producing characteristic attachment–effacement lesions on the enterocyte plasma membrane. Because EPEC colonizes the duodenum, it can be detected by culturing duodenal aspirates (304).
strain. Almost all deaths occur before age 2. The organisms are not invasive; nor do they produce toxins. They colonize the proximal small intestine via specific attachment mechanisms, producing characteristic attachment–effacement lesions on the enterocyte plasma membrane. Because EPEC colonizes the duodenum, it can be detected by culturing duodenal aspirates (304).
TABLE 6.14 Types of Pathogenic Escherichia coli | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Enterohemorrhagic E. coli (EHEC) cause hemorrhagic co-litis, hemolytic uremia syndrome, and thrombotic thrombocytic purpura; they are discussed in Chapter 13.
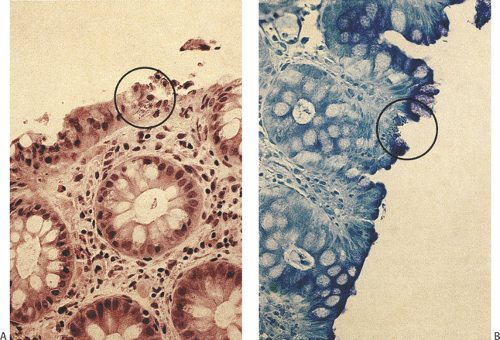
Enteroadherent E. coli are nontoxigenic and does not invade the mucosa (302). They avidly adhere to the epithelial brush border via specific receptors (Fig. 6.132). Often the histology of the small intestine in infected individuals is normal. Children with this infection develop chronic prolonged diarrhea (305). Rarely, villous atrophy results. The lesions may resemble celiac disease.
Enterotoxigenic E. coli (ETEC) elaborate an enterotoxin similar to cholera toxin (306). They represent a major cause of traveler’s diarrhea and infant diarrhea in underdeveloped countries. The disease is initiated by consumption of contaminated food or water or by contact with infected persons. Once the bacteria are ingested, they pass to the small bowel, where they colonize the surface epithelium. ETEC adhere to enterocyte brush borders without damaging them. The adhesion is specific, and pili and fimbrial colonization factor antigens determine host specificity. Once in the small intestine, the bacteria produce two toxins: A heat-labile toxin resembling cholera toxin and a heat-stable toxin that activates guanylate cyclase and stimulates active fluid secretion without injuring the enterocytes (307). The disease often begins with upper intestinal distress followed by watery diarrhea. The clinical course may be extremely mild or very severe, mimicking cholera and producing severe dehydration and rice water stools. Symptoms consist of the sudden onset of abdominal cramps, nausea, borborygmi, and malaise. Acute watery diarrhea then develops, followed by dehydration with low-grade fever and chills.
The presenting symptoms of enteroinvasive E. coli (EIEC) infection include diarrhea, tenesmus, fever, and cramps. The organism penetrates and multiplies within epithelial cells. Clinically, most patients manifest watery, nonbloody diarrhea. This organism is unusual in the United States, although it is relatively common in Thailand (308).
Enteroaggregative E. coli (EAEC) have been implicated as an important agents of persistent diarrhea among infants in the developing world and as a cause of traveler’s diarrhea. A plasmid encodes the gene for the aggregative adherence
properties of EAEC. Ultrastructurally, one can show that the EAEC strains have four morphologically distinct kinds of fimbriae that mediate cellular adhesion and induce mucosal damage (309). The typical illness presents with a watery mucoid secretory diarrhea with low-grade fever and little or no vomiting. The diarrhea may last for several weeks. The disease is diagnosed by isolating the organism from the stool and the demonstration of an aggregative adherent pattern on HEp-2 assay.
properties of EAEC. Ultrastructurally, one can show that the EAEC strains have four morphologically distinct kinds of fimbriae that mediate cellular adhesion and induce mucosal damage (309). The typical illness presents with a watery mucoid secretory diarrhea with low-grade fever and little or no vomiting. The diarrhea may last for several weeks. The disease is diagnosed by isolating the organism from the stool and the demonstration of an aggregative adherent pattern on HEp-2 assay.
Salmonella Infections
Salmonella organisms are Gram-negative bacilli that produce five clinical syndromes: (a) gastroenteritis (70% of infections); (b) bacteremia with or without GI involvement (10%); (c) typhoid or enteric fever; (d) localized infections in joints, bones, and meninges (5%); and (e) an asymptomatic carrier state. Most Salmonella species can produce any of these syndromes, but certain serotypes more commonly associate with specific clinical presentations (310). Usually, Salmonella causes a mild self-limited illness, but very young, elderly, and immunocompromised patients develop serious complications, sepsis, and death. The disease pattern reflects the inherent bacterial virulence, the number of organisms ingested, their ability to survive and/or replicate, the presence of a normal flora in the upper intestinal tract, and host status. Salmonella species are generally divided into typhoid and nontyphoid species.
Infection with Salmonella typhi is almost always transmitted person to person (311). Infections with most other Salmonella species, except for Salmonella paratyphi, derive from environmental sources, principally poultry and livestock. The current rarity of typhoid fever in the United States reflects good hygiene, lack of crowding, and high public standards for home and industrial sewage.
Typhoid fever is spread by contaminated food or water. Humans are the only known reservoir for S. typhi, which is transmitted via a fecal–oral route. Its annual incidence in the United States is 0.2 cases per 100,000 population. Higher incidence rates occur in areas with contaminated water supplies and inadequate waste disposal. The usual victims are children and young adults. However, recently a nosocomial outbreak of fluoroquinolone-resistant Salmonella enterica was described among elderly individuals in a nursing home (312). Typhoid fever may also be sexually transmitted among men having sex with men (312a).
Patients usually remain relatively asymptomatic during an incubation period of a week followed by a 2- to 3-week period of illness. Then the patients present with fever, abdominal pain, and headache. Abdominal rash, delirium, hepatosplenomegaly, and leucopenia are common. Diarrhea begins in the second and third weeks of the infection. It is watery at first but may become bloody, and perforation may occur. Typhoid fever is a chronic systemic illness with a mortality rate of 15% in untreated individuals and 1% in treated individuals. A prolonged convalescent stage of 3 months is usual. GI complications include perforation, massive intestinal hemorrhage (4% to 7%), peritonitis, and paralytic ileus (313). Perforation causes death in 25% to 33% of patients who die of the disease (314). If the patient recovers, intestinal lesions heal with minimal fibrosis so that stricture formation is unusual.
Nontyphoid species (Salmonella enteritidis, Salmonella typhimurium, Salmonella muenchen, Salmonella anatum, S. paratyphi, and S. give) result in a milder, more self-limited gastroenteritis with nausea, vomiting, fever, and watery diarrhea.
Salmonella organisms are facultative intracellular parasites capable of penetrating, invading, surviving, and multiplying in many cells. Plasmids encode the virulence factors involved in adherence to, invasion of, and growth within the epithelium (315). Salmonella organisms adhere to M-cell and enterocyte surfaces, where they induce almost complete destruction (316) as described in Chapter 13. When two or more cells are destroyed, the resulting mucosal defect predisposes to deeper infection (317). If bacteria pass between enterocytes, the tight junctions separate and then reseal following bacterial passage. Internalization of Salmonella is receptor mediated, possibly involving the epidermal growth factor receptor> Once inside the cells, the bacteria become enclosed by membrane-bound cytoplasmic vacuoles and they then multiply. Following their release from the vacuoles, they disseminate to the regional lymph nodes, spleen, and liver, sites where the bacteria multiply further (316).
Salmonella may infect any part of the gastrointestinal tract, but the ileum, appendix, and right colon are preferentially affected. The bowel wall appears thickened. Swollen, raised, ulcerated Peyer patches produce the typical gross appearance of small, longitudinally oriented (oval) terminal ileal ulcers (Fig. 6.133). Other ulcers appear aphthous, linear, discoid, or full thickness in nature. Ulcers are less prominent in the proximal small bowel. With progressive bowel wall involvement, it becomes paper thin and susceptible to perforation. In some cases, the bowel may appear grossly normal or only mildly erythematous in nature.
Shortened edematous villi contain neutrophilic infiltrates coexisting with crypt hyperplasia. There is marked vascular
congestion and lymphoplasmacytic infiltrates along with histiocytes with abundant eosinophilic cytoplasm containing nuclear debris and red blood cells. Peyer patches become hyperplastic (Fig. 6.134) followed by acute inflammation of the follicle-associated epithelium. Edema, fibrinous exudates, and vascular thromboses herald the onset of tissue necrosis and ulceration, causing the lymphoid follicles to appear elevated over the mucosal surface (Fig. 6.135). As the follicle-associated epithelium ulcerates, the lymphoid tissue extends from the submucosa to the mucosal surface, discharging large numbers of bacilli into the intestinal lumen. Eventually the lymphoid follicles become infiltrated and obliterated by macrophages. Architectural distortion severe enough to mimic irritable bowel disease (IBD) may be present.
congestion and lymphoplasmacytic infiltrates along with histiocytes with abundant eosinophilic cytoplasm containing nuclear debris and red blood cells. Peyer patches become hyperplastic (Fig. 6.134) followed by acute inflammation of the follicle-associated epithelium. Edema, fibrinous exudates, and vascular thromboses herald the onset of tissue necrosis and ulceration, causing the lymphoid follicles to appear elevated over the mucosal surface (Fig. 6.135). As the follicle-associated epithelium ulcerates, the lymphoid tissue extends from the submucosa to the mucosal surface, discharging large numbers of bacilli into the intestinal lumen. Eventually the lymphoid follicles become infiltrated and obliterated by macrophages. Architectural distortion severe enough to mimic irritable bowel disease (IBD) may be present.
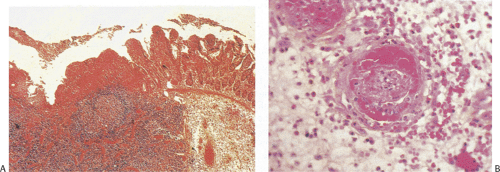
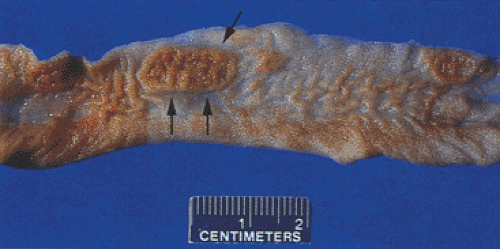 FIG. 6.133. Salmonella enteritis. Note the presence of the prominent longitudinal ulcer overlying the Peyer patches (arrows). |
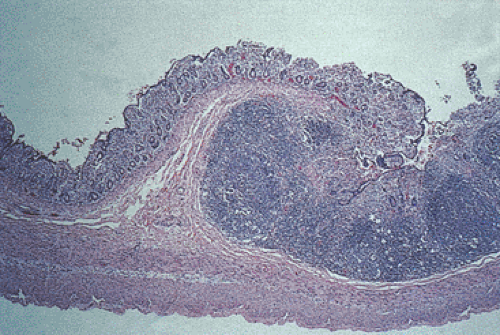 FIG. 6.134. Histologic lesion of the specimen shown in Figure 6.133 showing prominent lymphoid hyperplasia in the Peyer patches surrounded by atrophic small intestine and demonstrating superficial ulceration with surrounding regeneration. |
The proliferation of monocytic elements is arranged in both a diffuse and nodular pattern. The nodular areas lie at the periphery of the lesions and the diffuse proliferations are in the center. The nodules are of two types. One contains a germinal center and consists of a mixture of centrocytes, centroblasts, and macrophages surrounded by a ragged compressed mantle zone. The second and predominant type of nodule consists of uniform sheets of monocytes/ macrophages, many containing numerous apoptotic bodies and cellular debris rimmed by small lymphocytes. Some of these areas may contain foci of amorphous eosinophilic debris and cells with peripherally displaced nuclei. The interfollicular and diffuse areas are dominated by phagocytic macrophages with a round to irregular (occasionally crescenteric) shape and also contain intermingled small, mature-appearing lymphocytes. Occasional plasma cells and immunoblasts are also present. Neutrophils are surprisingly rare, even in the areas of ulceration. This inflammatory reaction extends into the muscularis propria and may even reach the serosa. Small mucosal erosions in areas of nonthickened bowel wall also consist of lymphoid infiltrates with germinal center formation and clusters of monocytes/macrophages, likely representing the early lesions (318).
The regional lymph nodes may show a necrotizing lymphadenitis and a marked sinusoidal and paracortical expansion due to a proliferation of monocytes/macrophages identical to those present in the involved bowel segments. Subcapsular sinuses are distended by monocytes in many areas and compressed or obliterated centrally. Actively phagocytic macrophages are present, many of which contain apoptotic fragments. The necrotic areas are rounded rather than stellate in shape and are rimmed by foamy macrophages that blend smoothly with the cellularity in the remainder of the lymph node (318).
The closest mimic of these changes are induced by Yersinia infections. Both diseases center around the ileocecal region and both the mucosa and the regional lymph nodes are distorted by lymphoid and histiocytic hyperplasia.
However, penetrating ulcers and epithelioid granulomas only occur in Yersinia and the areas of necrosis in Yersinia are usually stellate in shape rather than being rounded.
However, penetrating ulcers and epithelioid granulomas only occur in Yersinia and the areas of necrosis in Yersinia are usually stellate in shape rather than being rounded.
Staphylococcal Infections
Staphylococcal food poisoning is an explosive but self-limited gastroenteritis associated with food poisoning. Staphylococci cause acute diarrheal disease by their ability to produce exotoxins that act as potent enterotoxins. In severe cases, patients have nonspecific enterocolitis with intense mucosal congestion, necrosis, and ulceration (319). Viable bacteria are absent.
Campylobacter Infections
Campylobacter species are spiral and highly motile Gram-negative rods> Numerous species exist within the genus Campylobacter, although classically only three are pathogenic for humans: Campylobacter jejuni, Campylobacter coli, and Campylobacter fetus. The frequency of the different Campylobacter infections differs from one country to another. Campylobacter is one of the most frequently isolated enteric stool pathogens. In one study it was found in 134 cases per 100,000 persons with diarrhea (320). The incidence in the United States is highest in children under 1 year of age and in persons 10 to 29 years of age (321). Because the disorder affects young adults, the initial clinical picture is easily confused with ulcerative colitis or Crohn disease. Water-borne outbreaks of Campylobacter enteritis associate with municipal water systems and with surface water. C. jejuni is a common cause of traveler’s diarrhea and of diarrheal illnesses suffered by hikers who drink untreated water in mountainous areas. Currently, Rocky Mountain Campylobacter enteritis occurs three times more frequently than does giardiasis (322). The disease incidence is highest in the summer. Outbreaks also associate with consumption of contaminated milk, meat, poultry, and vegetables (323); person-to-person transmission; and transmission from pets. Infected chickens currently account for 70% of disease risk (323).
Campylobacter produces several disease patterns: (a) asymptomatic infections, (b) acute enteritis, and (c) acute colitis; the disease pattern reflects the type of organism infecting the patient. For example, C. jejuni is more likely to cause an acute self-limited gastroenteritis, whereas C. fetus causes systemic illnesses that are often fatal. Acute enteritis is the most common presentation. The incubation period between organism ingestion and symptom onset ranges from 1 to 7 days. A typical episode begins with fever and malaise followed within 24 hours by nausea, vomiting, abdominal pain, and diarrhea. Enlarged mesenteric lymph nodes become symptomatic in some patients (324). Grossly bloody stools are common, and many patients have at least 1 day with eight or more bowel movements. Headache and myalgia, which tend to be more severe than in other forms of infective enterocolitis, develop in some patients. Severe complications, including sepsis, meningitis, toxic megacolon, pseudomembranous colitis, massive GI hemorrhage, arthritis, endocarditis, and genital and urinary infections, affect debilitated individuals. However, the illness is usually self-limited. Twenty percent of patients experience a relapse. The organism also causes death (325), especially in immunocompromised persons. A complication of Campylobacter infection is the Guillain-Barré syndrome. It results from cross-reactivity between neural and C. jejuni antigens (326). Most patients recover fully from their infections, either spontaneously or after antibiotic therapy.
Campylobacter enterocyte adhesion is critical to the development of the enteritis. When the organisms invade the cell surface, the epithelium becomes damaged and bacteria invade the cells. The organism also produces toxins that bind to specific receptors on cell membranes, damaging the cells (327).
Endoscopic features of Campylobacter infections include patchy mucosal inflammation, hyperemia, bloody exudates, segmental mucosal edema, loss of a normal vascular pattern, and ulceration. The changes mimic inflammatory bowel disease. Grossly, one sees a diffuse, bloody, edematous enterocolitis with multiple, superficial ileal ulcers measuring up to 1 cm in diameter just proximal to the ileocecal valve and involving the valve itself. The ulcers sometimes coincide with the region of Peyer patches.
Biopsies of both the enteritis and the colitis show nonspecific acute and chronic changes resembling other infections. Small intestinal lesions include inflammation and edema of the lamina propria, neutrophilic infiltrates, goblet cell depletion with crypt abscesses, and hemorrhagic necrosis. Other histologic features include hemorrhagic ulcers, erythema, cryptitis, broadening and flattening of the villi, mucosal atrophy, and features similar to those seen in chronic ulcerative colitis, nonspecific colitis, or infective colitis. The ulcer bases have acute inflammation, granulation tissue, and prominent vascularity, but they lack fibrosis (328). Rectal lesions are also often present. Unlike chronic ulcerative colitis, the changes tend to be segmental in nature. Poorly formed mucin granulomas can be seen. Suppurative foci in the granulomas distinguish the lesion from Crohn disease. If tissue is examined during the necrotizing phase, the epithelium will appear regenerative in nature. Because biopsies are usually nonspecific, bacterial culture or other diagnostic techniques are usually necessary to establish the diagnosis.
Vibrio Infections
Eleven Vibrio species cause human infections (329,330,331,332,333,334), but most infections result from Vibrio parahaemolyticus, Vibrio cholerae, Vibrio vulnificus, and Vibrio cholerae. Vibrio organisms are motile, Gram-negative, curved, rod-shaped bacteria, living in marine or brackish water. The seasonality of cholera relates in part to the presence of viable, noncultivatable forms of the organism in marine life and algae. In this environment, V. cholerae assume sporelike forms; when they are re-exposed to a more favorable environment, they revert to an infectious
state. The sporelike form of Vibrio is consumed by fish, mollusks, and crustaceans. Infections with V. vulnificus commonly occur among persons who work in the shellfish industry, especially among individuals who sustain a puncture wound while handling fresh seafood. V. parahaemolyticus infections are a major cause of food poisoning, transmitted by sashimi or infected oysters. Rising ocean temperature waters appear to be contributing to continuing disease outbreaks (335).
state. The sporelike form of Vibrio is consumed by fish, mollusks, and crustaceans. Infections with V. vulnificus commonly occur among persons who work in the shellfish industry, especially among individuals who sustain a puncture wound while handling fresh seafood. V. parahaemolyticus infections are a major cause of food poisoning, transmitted by sashimi or infected oysters. Rising ocean temperature waters appear to be contributing to continuing disease outbreaks (335).
V. cholerae infections may be mild or may be an extremely deadly disease. Disease virulence relates to the genes that encode the toxin responsible for cholera’s life-threatening diarrhea. Cholera enterotoxin is the prototype for secretory enteritis and toxigenic diarrhea. The cholera toxin induces elevated mucosal levels of cyclic adenosine monophosphate (AMP). This has a direct secretory effect on crypt cells and an antiabsorptive effect on villous cells, producing the most overwhelming feature of the disorder: extreme fluid loss.
The clinical spectrum of cholera varies from asymptomatic carriers to patients with extremely severe diarrhea. In the acute phase, water secretion from the small intestine is greater than the capacity of the colon to absorb the water loss, resulting in large fluid, sodium, potassium, and bicarbonate losses. Cholera is a leading cause of death in some parts of the world because the large volume depletion leads to acidosis and renal failure. Death can occur within 3 to 4 hours of onset, with fecal output exceeding 1 L/hr at the height of the disease. Daily outputs of 15 to 20 liters have been observed when adequate fluid replacement is given. Incubation periods range from 6 to 48 hours and recovery occurs in 2 to 7 days. Untreated, the mortality ranges between 50% and 75% (336).
Although cholera is widespread, the histopathologist plays less of a role in its diagnosis than the microbiologist. Grossly, the bowel appears slightly edematous and only mildly abnormal. The intestinal mucosa appears intact with mucus-depleted dilated crypts. If goblet cells are present, they appear empty, and the lamina propria exhibits mild edema and vascular dilation. Occasional inflammatory cells are present but significant inflammation is absent. Widening of intercellular spaces is most prominent in the villous epithelium, whereas blebbing of microvillous border and mitochondrial changes usually occur in the crypts (337).
Three major clinical syndromes result from the V. vulnificus infections: Primary septicemia, wound infections, and GI illness without septicemia or wound infection (338). The disease is characterized by a 24-hour incubation period followed by the sudden onset of septicemia, fever, chills, hypotension, nausea, vomiting, bloody diarrhea, and a rash. Infection symptoms and duration are not as severe as in cholera. Skin lesions are distinctive. Large hemorrhagic bullae progress to necrotic ulcers.
Aeromonas Infections
Aeromonas organisms are facultative anaerobic Gram-negative bacteria ubiquitous in fresh and brackish water and soil. The infection may also be acquired through consumption of untreated water or trauma, usually involving soil exposure in individuals with lacerations on the extremities. The organism has been isolated from children with acute diarrhea in daycare centers and from adults with traveler’s diarrhea. The disease also affects neonates. Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae cause a spectrum of GI diseases, ranging from a self-limited diarrhea to acute persistent dysentery (339). Typical symptoms include low-grade fever, watery stools often with occult blood, and abdominal cramps. Children present with severe diarrhea, whereas the adult disease tends to be chronic in nature with patients presenting with vomiting and abdominal cramps. Aeromonas produces enterotoxins, cytotoxins, and hemolysins. Histologic changes range from changes that resemble celiac disease, to edema of the lamina propria, to a full-blown enterocolitis (Fig. 6.136). The diagnosis is made by stool culture.
Plesiomonas Infections
Plesiomonas shigelloides, a facultative anaerobic Gram-negative rod-shaped bacterium, is recoverable from the stool in up to 17% of individuals with diarrhea (339). It associates with eating uncooked fish and with foreign travel, usually to Mexico. Most infected individuals experience a self-limited diarrhea with blood and mucus in the stool. Rarely, severe enterocolitis, pseudoappendicitis, osteomyelitis, and bacteremia develop.
Clostridial Infections
Various clostridial strains normally inhabit the large bowel and occasionally the ileum. Because clostridial infections more commonly involve the colon than the small bowel, the infections are discussed in Chapter 13. However, there are several unique forms of small intestinal clostridial diseases that will be covered in this chapter.
Enteritis Necroticans
Enteritis necroticans is a life-threatening infectious disease caused by Clostridium perfringens type C, a β-toxin–producing strain of clostridia causing necrosis and sepsis (340). The disease is characterized by segmental necrosis of the proximal jejunum and is associated with a high mortality rate if not diagnosed and treated early. It was first reported in Northern Germany after World War II among previously starved children and adults who ate large meals of meats and vegetables. The disease rarely occurs in developed countries; when it does it typically affects diabetics (341).
This disorder is also known as Darmbrand enteritis (342), necrosis jejunitis, or jejunitis acta. The lesion starts abruptly in the proximal jejunum and usually extends distally into the ileum. It presents as abdominal pain, vomiting, diarrhea, and progressive dehydration. The affected bowel becomes dilated,
edematous, thickened, rigid, and markedly congested. Valvulae conniventes become very prominent, imparting a washboard appearance to the mucosal surface. When the necrotic mucosa sloughs, extensively ulcerated areas remain. More severe cases have transmural inflammation.
edematous, thickened, rigid, and markedly congested. Valvulae conniventes become very prominent, imparting a washboard appearance to the mucosal surface. When the necrotic mucosa sloughs, extensively ulcerated areas remain. More severe cases have transmural inflammation.
Histologically, one sees severe mucosal necrosis with hemorrhage, mural thickening due to marked submucosal edema, and fibrinous or fibrous serosal exudate. Pseudomembranes and fibrin thrombi may be present in the inflamed areas. The mucosa between the thickened valvulae may appear normal, whereas the surfaces of the valvulae are more damaged. This pattern suggests a clostridial infection. There is little in the way of a neutrophilic response (342).
Pig Bel
A similar disease, pig bel, is endemic in Papua, New Guinea. It results from Clostridium welchii and C. perfringens infections (343), and it is the most common cause of death in children. Individuals become ill from eating infected pigs during ceremonial occasions. Patients present with symptoms lasting for a few days. These consist of severe, progressive abdominal pain, vomiting, blood-stained feces, and constipation. The small intestine shows segmental necrosis starting at the villous tips and progressing toward the base. The disease affects the duodenum to cecum. Alternatively, multiple segments of small intestine are involved, separated by normal-appearing unaffected segments. Kinking and adhesions between adjacent loops of bowel affect 50% of cases. At the time of surgery, the small intestine appears dilated and the distended bowel has a thickened wall and a red serosal surface. In about 25% of cases one or more yellow patches exist on the serosal surfaces. These represent areas of full-thickness infarction (Fig. 6.111).
Histologically, one sees ischemic changes with necrotic villi. Numerous bacteria are present. A clearly defined junctional zone exists between the necrotic mucosa and the submucosa. The vessels at this junction are thrombosed. Neutrophils accumulate at the edges of infarction. The mucosa becomes infiltrated with neutrophils, mononuclear cells, and eosinophils. Arterial walls appear edematous and infiltrated by inflammatory cells, and they demonstrate a homogeneous or granular eosinophilic appearance classically linked with fibrinoid necrosis.
Yersinia Infections
Yersinia enterocolitica (YE) and Yersinia pseudotuberculosis (YP) are facultative, anaerobic, non–lactose-fermenting, Gram-negative coccoid bacilli. The incidence of infection is highest in the cold months and in cold climates. YE and YP are a common cause of bacterial enteritis in Western and Northern Europe, but increasing numbers of cases have been reported in North America and Australia. Transmission usually follows ingestion
of contaminated meats, vegetables, water, and milk (344). Swine constitute an important reservoir for human infections. However, the organism has also been isolated from numerous other animals. Transmission also occurs from dogs and cats and from person-to-person transmission via blood transfusion. Belgium is the country with the highest reported increase of the disease, strongly correlating with the consumption of raw pork. Most infections are self-limited. However, immunocompromised and debilitated patients as well as patients with iron overload are at a risk of serious disease.
of contaminated meats, vegetables, water, and milk (344). Swine constitute an important reservoir for human infections. However, the organism has also been isolated from numerous other animals. Transmission also occurs from dogs and cats and from person-to-person transmission via blood transfusion. Belgium is the country with the highest reported increase of the disease, strongly correlating with the consumption of raw pork. Most infections are self-limited. However, immunocompromised and debilitated patients as well as patients with iron overload are at a risk of serious disease.
The pathogenicity of Yersinia species is determined by a number of virulence factors encoded by the bacterial chromosomes or by the virulence plasmid pYV (345,346). Chromosomally encoded proteins facilitate bacterial intestinal attachment, mucosal penetration, survival, and proliferation. Virulence factors include various adhesins, invasin, and attachment-invasion locus. In addition, the pYV proteins make up a type III secretion system similar to that seen in pathogenic E. coli and Salmonella. A “high pathogenicity island” (HPI) is present in only highly pathogenic strains of Yersinia. The Yersinia HPI carries a cluster of genes involved in the biosynthesis, transport, and regulation of the siderophore yersiniabactin. The major function of the island is to acquire the iron molecules essential for bacterial growth and dissemination (347). Luminal bacteria adhere to M cells or absorptive cells in areas of the follicle-associated epithelium. Once inside the cell, the bacteria become enclosed by membranous vesicles (348). YE penetrate into the lamina propria by passing through the enterocyte cytoplasm in a manner similar to that exhibited by Salmonella. Only potentially pathogenic strains invade the lamina propria. YE organisms multiply within lymphoid follicles in Peyer patches, then drain into the mesenteric lymph nodes, eventually giving rise to systemic infections.
Yersinia preferentially involves the ileocecal and appendiceal regions and causes a wide spectrum of clinical and pathologic changes, ranging from self-limited enterocolitis to potentially fatal systemic infections. Diffuse enterocolitis, the most common clinical manifestation, usually affects children younger than 5 years of age (349). Patients present with gastroenteritis, diarrhea, low-grade fever, abdominal pain, ileitis, colitis, diffuse mesenteric lymphadenitis, pseudoappendicitis, sepsis (350,351), intestinal perforation, and the hemolytic uremia syndrome. Older children and adults develop mesenteric adenitis mimicking acute appendicitis. Immunosuppressed hosts often develop severe, fatal, Yersinia bacteremia. Patients who are in iron-overloaded states such as those with hemochromatosis or transfusional siderosis develop particularly virulent infections (352). Postinfection manifestations result from bacteremia and include erythema nodosum, bullous skin lesions, and reactive arthritis.
Grossly, Yersinia infections mimic Crohn disease. The bowel wall appears thickened, inflamed, congested, ulcerated, and edematous. Patients develop diffuse, focal, or aphthous mucosal ulcers. Even though numerous ulcers may be present, they are usually not very deep. The intestinal serosa appears dull and hyperemic (Fig. 6.137). The muscularis propria thickens. Massively enlarged lymph nodes may become matted, and they may contain yellowish microabscesses. The enlarged mesenteric lymph nodes may become matted and contain yellowish microabscesses. Both the small and large intestines are involved, with the most severe changes centering on the ileocecal region and appendix.
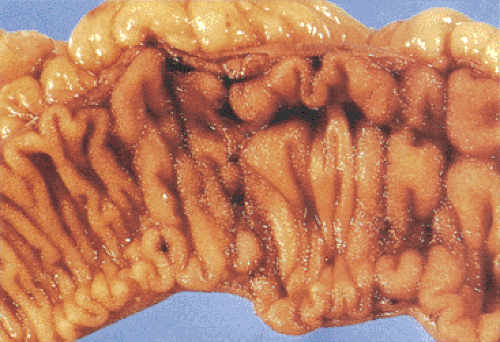 FIG. 6.137. Yersinia. Gross appearance in a patient with culture-proven Yersinia infection. The mucosal folds are unduly prominent due to the presence of the granulomas. |
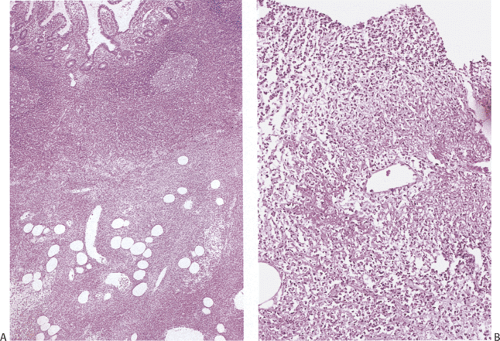
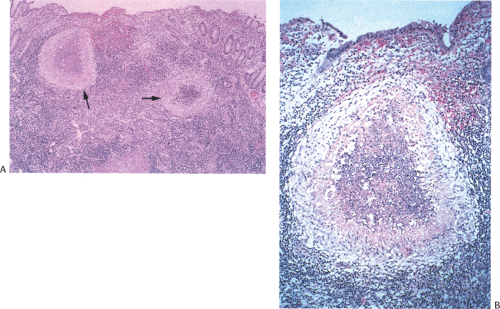
There is considerable overlap in the histologic features of YE and YP. Both infections can produce mucosal ulceration, cryptitis, granulomatous inflammation with prominent lymphoid cuffing, lymphoid hyperplasia, transmural lymphoid aggregates, and nodal involvement. The affected bowel appears congested, edematous, and ulcerated with massively enlarged lymphoid follicles. Sharply demarcated areas of lymphoid hyperplasia contain prominent germinal centers. The follicular ileitis may persist for months. The mucosa overlying the follicles develops small punctate aphthoid ulcers measuring 1 to 2 mm in diameter (351) resembling similar lesions in Crohn disease. These ulcers are covered by fibrinopurulent exudates and large numbers of Gram-positive coccobacilli. Sharply demarcated lymphoid hyperplasia is present with prominent germinal centers and small aphthoid ulcers overlying the hyperplastic lymphoid follicles. Epithelial granulomas with central necrosis may be seen in the bowel wall and in the lymph nodes. The bowel and lymph nodes contain epithelioid granulomas with central necrosis (Fig. 6.138). Granulomas are located in the mucosa and submucosa, but they can also occur on the serosa. The muscularis propria and serosa become infiltrated by pleomorphic cellular infiltrates including eosinophils. Acute vasculitis or intussusception may cause ischemia (Fig. 6.139). Crypt hyperplasia occurs throughout the small intestine with villous atrophy (351). Strictures are rare. The changes superficially resemble those found in cat-scratch fever.
The most important entity in the differential diagnosis is Crohn disease, which may be very difficult to distinguish from Yersinia infections histologically. Culture, serologic
studies, and polymerase chain reaction (PCR) assays may confirm Yersinia as the cause of the disease. Features that favor a diagnosis of Crohn disease include prominent neural hyperplasia and evidence of chronic changes including crypt distortion and hyperplasia of the muscularis mucosae.
studies, and polymerase chain reaction (PCR) assays may confirm Yersinia as the cause of the disease. Features that favor a diagnosis of Crohn disease include prominent neural hyperplasia and evidence of chronic changes including crypt distortion and hyperplasia of the muscularis mucosae.
Tuberculosis
In the 1980s, after decades of steadily declining rates of tuberculosis, ambitious plans were made to eliminate the disease in the United States. However, despite these plans, the control of tuberculosis was neglected, resulting in a resurgence of the disease (353). Between the mid-1980s and the early 1990s, the synergistic combination of a deteriorating public health infrastructure, inadequate institutional control of infection, urban crowding, the HIV epidemic, and immigration resulted in a resurgence of tuberculosis including infections with multidrug-resistant strains.
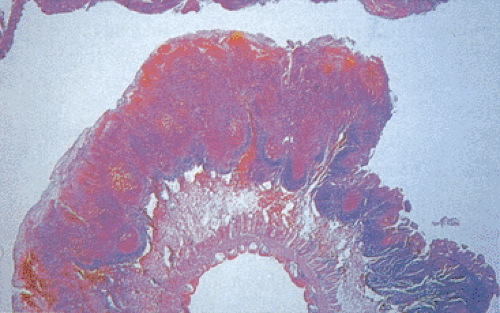 FIG. 6.139. Yersinia enteritis. Yersinia granulomatous enterocolitis causing intussusception and secondary ischemic necrosis. |
Tuberculosis is currently uncommon in North America but it remains endemic in Asia, where it constitutes a major health problem. In 2000, 16,377 cases (5.8 cases per 100,000 population) were reported to the Centers for Disease Control and Prevention (CDC), representing a 45% decrease in the incidence rate and the lowest rate in U.S. history (354). In the United States, 50% of all cases of tuberculosis occur in foreign-born individuals (355), who represent only 10% of the total population (356). Tuberculosis in the immigrant population is largely attributed to the importation of latent infection with subsequent reactivation of the disease (357). The rate of tuberculosis among immigrants who have lived in the United States for 5 years or less is three times as high as that among immigrants who have lived here for more than 5 years (358). Recognizing that existing public health practices are inadequate, both the CDC and the Institute of Medicine have called for stronger measures to detect and treat latent infection in immigrants to the United States (359). In addition to immigrants, HIV-infected persons, minorities, the homeless, incarcerated, alcoholics, and the poor have increased infection rates. Others at risk for the infection may
include hospital workers and the military. Blacks are more likely to contract tuberculosis than persons of other races (360). Recent developments in bacterial genotyping are providing a better understanding of the pathogenesis, transmission, drug resistance, and reinfection of tubercular infections (361).
include hospital workers and the military. Blacks are more likely to contract tuberculosis than persons of other races (360). Recent developments in bacterial genotyping are providing a better understanding of the pathogenesis, transmission, drug resistance, and reinfection of tubercular infections (361).
The severity of GI involvement relates to the severity of pulmonary infections and is most common in individuals with cavitary lung lesions and a positive sputum. Swallowed organisms pass through the small bowel mucosa without causing a local lesion, only to arrest in the regional lymph nodes. Ulcerative small intestinal lesions develop as the result of retrograde spread.
Complications of intestinal tuberculosis include severe enterocolitis, hemorrhage, perforation, obstruction, fistula formation, strictures, malabsorption, and severe secretory diarrhea. The latter results from bacterial overgrowth in dilated intestinal loops lying proximal to points of obstruction. It may also result from obstruction of the mesenteric lymphatics by granulomas in the regional lymph nodes. Chronic nonspecific abdominal pain is the most common complaint. Other findings include fever, weight loss, and malaise. The clinical differential diagnosis includes carcinoma, tuberculosis, Crohn disease, and Yersinia infections.
AIDS patients may develop generalized tuberculosis, and up to 50% of patients who die are undiagnosed premortem (362). This is due to the fact that generalized tuberculosis in the setting of AIDS often lacks the typical features of the disease. Inflammatory foci show areas of necrosis with numerous neutrophils, numerous acid-fast bacilli, few or no epithelioid histiocytes, and no Langhans giant cells (362).
The ileocecal region is affected in 90% of patients (Figs. 6.140 and 6.141); other affected locations in decreasing order of frequency include the ascending colon, appendix, jejunum, duodenum, stomach, sigmoid, and rectum. This distribution follows the distribution of the lymphoid tissues.
The area of the ileocecal valve is frequently obscured by a mass that consists of mesenteric fat, fibrotic tissue, lymph nodes, and other inflammatory changes. The appendix and distal terminal ileum are usually thickened and strictured, with mucosal ulceration and surface fibrin deposits. The wall of the colon may be so extensively destroyed by the inflammatory process that one cannot tell which side of the ileocecal valve one is on.
The area of the ileocecal valve is frequently obscured by a mass that consists of mesenteric fat, fibrotic tissue, lymph nodes, and other inflammatory changes. The appendix and distal terminal ileum are usually thickened and strictured, with mucosal ulceration and surface fibrin deposits. The wall of the colon may be so extensively destroyed by the inflammatory process that one cannot tell which side of the ileocecal valve one is on.
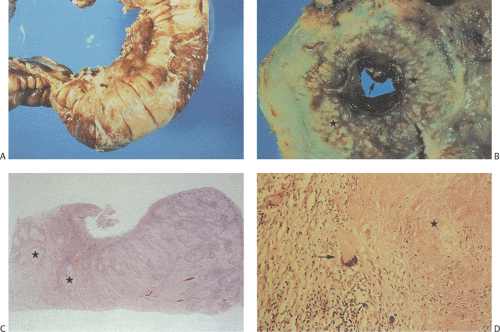
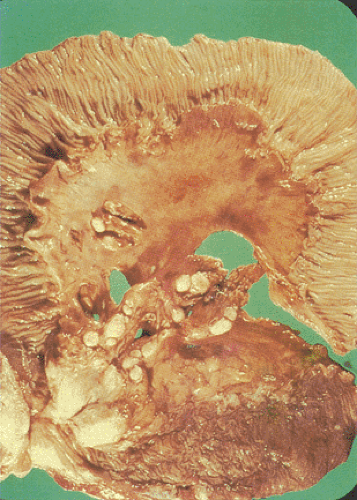
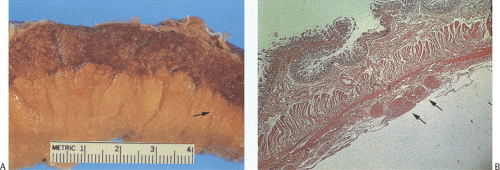
Classically, intestinal tuberculosis assumes one of three forms: (a) the ulcerative form (60% of cases), which exhibits multiple superficial ulcers and has a virulent course with a high mortality; (b) the hypertrophic form (10% of cases), which grossly mimics Crohn disease because of the scarring, fibrosis, and heaped-up mass lesions; and (c) the ulcerohypertrophic form (30% of cases), in which the intestinal wall becomes thickened and ulcerated by an inflammatory mass centering around the ileocecal valve (363). The cut surface of the bowel appears friable, and necrotizing granulomas are easily seen.
Tubercles always begin in Peyer patches or lymphoid follicles and may give the mucosa a cobblestoned appearance. As the disease progresses, tubercles encircle the entire bowel wall (Fig. 6.140). Multiple tubercles may also stud the serosa and mesentery (Fig. 6.142). The ulcerative form of the disease begins as ragged, undermining ulcers. The ulcers may be single or multiple, large or small (Fig. 6.143). In contrast to Crohn disease, tuberculous ulcers tend to be circumferential with their long axis perpendicular to the lumen without fissuring. The ulcers may contain acid-fast bacilli (AFB), even in the absence of granulomas. The nonulcerated mucosa usually appears markedly edematous and focally hemorrhagic. Hyperplastic lesions cause pronounced intramural thickening with ulceration and obstruction (Fig. 6.140). Fibrosis, strictures, and stenosis result when the ulcers heal. Areas of irregular stenosis may measure several centimeters in length. This leads to the hypertrophic form of the disease. Giant cell granulomas with obvious caseation occur more frequently in ulcerative than hyperplastic lesions. They are distributed throughout the entire thickness of the intestinal wall (Fig. 6.140). Although tuberculosis classically associates with granulomas, it is only one of several causes of ileocecal
granulomas (Table 6.15). Regional mesenteric lymph nodes become enlarged, containing areas of caseous necrosis (Fig. 6.144). Isolated organisms can be visualized in the tubercles and lymph nodes with the use of special stains, or they are recoverable from tissue culture.
granulomas (Table 6.15). Regional mesenteric lymph nodes become enlarged, containing areas of caseous necrosis (Fig. 6.144). Isolated organisms can be visualized in the tubercles and lymph nodes with the use of special stains, or they are recoverable from tissue culture.
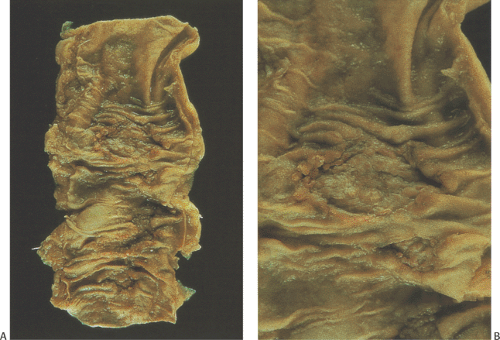 FIG. 6.143. Tuberculous enteritis. A: Resection specimen demonstrating the presence of the ulcerative form of tuberculosis. B: The ulceration at higher magnification. |
TABLE 6.15 Intestinal Granulomas | |
|---|---|
|
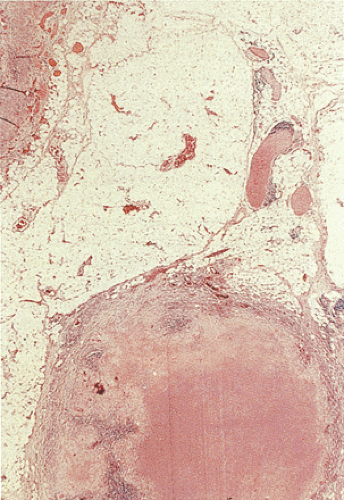 FIG. 6.144. Histologic section of the lymph node removed from the resection specimen shown in Figure 6.143. The lymph node is almost completely replaced by a caseating granuloma. |
Sometimes it is difficult to detect the bacteria in suspected cases of tuberculosis, even in AFB-stained sections, due to the scarcity of the organisms. In this setting the detection of mycobacterial DNA in formalin-fixed paraffin-embedded tissue by duplex PCR reactions may prove valuable in the management of patients with granulomatous enterocolitis (364).
Mycobacterium avium-intracellulare Infections
Mycobacterium avium-intracellulare (MAI), a highly prevalent (ubiquitous) AIDS-associated pathogen, affects 15% to 40% of AIDS patients. Persons acquire the organisms through environmental exposure to aerosols, water, food, and soil. Unlike tuberculosis, which appears to result from reactivation of a previously contracted infection, disseminated MAI usually results from a primary infection. A period of asymptomatic colonization of the respiratory or GI tract often precedes disseminated disease, suggesting that the organism is acquired from the environment via the gut and then disseminates. The GI tract is involved twice as frequently as the lungs. Disseminated disease usually follows the primary infection within months.
Healthy people who become colonized by MAI do not normally develop active disease. The major risk factor for the infection is the level of immune dysfunction, as reflected by the CD4+ cell count. Between 76% and 90% of MAI infections develop late in the course of AIDS when other AIDS-defining diagnoses have already been established and CD4 counts measure <60/mm3. MAI may be excreted in the stool of AIDS patients without GI symptoms.
Virulence factors for MAI are not well established. The organisms penetrate the gastrointestinal mucosa by unknown mechanisms and are phagocytosed by macrophages in the lamina propria. The macrophages cannot kill the bacteria and they become stuffed with mycobacteria as they multiply within the cells. With continued bacterial replication the host cell ruptures. The process leads to the presence of sheets of macrophages laden with AFB. The bacteria spread through the submucosal tissues, where the lymphatic drainage carries them to the regional lymph nodes. In the lymph nodes, the same process is again repeated. The mycobacteria replicate in macrophages, eventually breaking loose and again forming sheets of infected cells that eventually replace the normal histology of the lymph node. Tissue destruction is rare and most signs and symptoms of MAI result from the elaboration of cytokines (365).
Symptomatic MAI infections typically present indolently with nausea, chronic diarrhea, abdominal pain, malabsorption resembling Whipple disease, fever, sweats, chills, weight loss, lymphadenopathy, hepatosplenomegaly, and pancytopenia. Obstructive jaundice develops secondary to massive involvement of the peripancreatic and porta hepatis lymph nodes. Intestinal obstruction may result from intussusception secondary to hyperplasia of Peyer patches and lymph node involvement (366). Death may result from malnutrition or superinfections. MAI also produces clinical and radiologic pictures resembling Crohn disease, especially in patients with terminal ileitis. At the time of autopsy, up to 60% of patients with MAI infection have GI involvement, even though the organism is rarely recognized antemortem (366). Numerous bacilli are seen in acid-fast–stained sections, both within macrophages and extracellularly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree