Fig. 43.1
Open lateral sphincterotomy. a Radial skin incision distal to the dentate line exposing the intersphincteric groove. b Elevation and division of the internal sphincter. c Primary wound closure. (With permission from [67] © Springer)
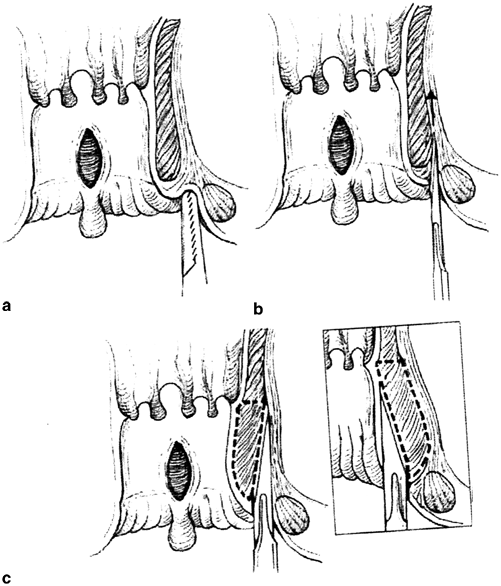
Fig. 43.2
Closed lateral internal sphincterotomy. a Location of the intersphincteric groove. b Insertion of the knife blade in the intersphincteric plane. c Lateral to medial division of the internal anal sphincter (inset: medial to lateral division of the muscle). (With permission from [67] © Springer)
Healing rates for CAF following LIS have been reported as high as 95 % [28–30]. Reported incontinence rates vary from 0 to 50 % [27, 30–34]. This wide variance had been attributed to multiple factors including surgical technique (open versus closed), length of LIS, type of anesthesia (local versus general), previous anorectal surgery, obstetric history, and inadvertent injury to EAS (Table 43.1) [9]. Perhaps the most important factor underlying the wide variation of reported incontinence rates following sphincterotomy is methodology of assessing the outcomes. Common methodological deficiencies include lack of clear definition of incontinence, failure to include the number of patients operated versus those surveyed, inadequate or poorly defined length of follow-up, use of nonstandardized or nonvalidated questionnaires, and failure to use nonbiased, objective examination of sphincter function. Of note, a recent Cochrane review (2011) evaluating the operative procedures for fissure-in-ano concluded that the combined analyses of open versus closed LIS show little difference between the two procedures in fissure persistence and risk of incontinence [35]. However, in regard to short- and long-term follow-up, Nyam et al. (1999) reported a 45 % incontinence in the short-term outcome, decreasing to < 11 % in long-term follow-up [33]. Lewis et al. (1988) reported a 17 % incontinence rate. This was only temporary in two-thirds of these patients [25]. The overall risk of incontinence in randomized surgical trials is reported to be about 10 % and is mostly incontinence to flatus [36].
Table 43.1
Factors responsible for wide variance noted in incontinence rates following LIS
Surgical technique (open versus closed) |
Length of LIS |
Type of anesthesia (local versus general) |
Previous anorectal surgery |
Obstetric history |
Inadvertent injury to EAS |
A 2012 meta-analysis identified subsets of individuals more prone to continence disturbances after sphincterotomy for fissure. These include age over 40, female gender, history of vaginal delivery, anterior fissure, addition of a synchronous anorectal procedure, and operative technique (Table 43.2) [15]. Preoperative anal manometry and endoanal ultrasonography should be considered in those high-risk patients to help delineate and define any possible preexisting sphincter injury and associated sphincter weakness.
Age over 40 |
Female gender |
History of vaginal delivery |
Anterior fissure |
Synchronous anorectal procedure |
Operative technique |
Murad-Regadas et al. (2013) conducted a prospective observational cohort study to determine the proportion of the IAS that may be divided during LIS in continent women without predisposing them to fecal incontinence [37]. 3D-endo anal ultrasound was used to evaluate the extent of the surgically divided portion of the IAS. Postoperative continence was objectively assessed via the Cleveland Clinic Florida score. They found that follow-up continence scores were significantly correlated with the extent of sphincter division. Continence was significantly better in those women whose sphincter division was less than 25 % versus those women with division of 25 % or more. Garcia-Aguilar et al. (1998) found that the IAS defects were wider in patients with incontinence than in those who were continent but this was not significant [38].
Fistulotomy
The goal of surgical treatment for anal fistula is eradication of the fistula tract without compromising sphincter function. Fistulas can be classified as “simple” or “complex.” “Simple” fistulas are of cryptoglandular infection, are usually distal intersphincteric or distal transsphincteric and can be treated by lay open fistulotomy with reported success rates reported over 90 % [39]. Those “simple” fistulas that involve proximal (high) intersphincteric or transsphincteric tracks are more difficult to manage. Fistulotomy of such proximal anal fistulas is associated with lower healing rates and higher rates of incontinence. “Complex” fistulas including those arising from noncryptoglandular origin such as those associated with perianal Crohn’s disease, those persisting or recurring despite prior surgical interventions, and those of cryptoglandular origin that crosses > 30–50 % of the external sphincter, are anterior in a female, are associated with multiple tracts, develop in an individual with some degree of existing fecal incontinence, or occur in previously irradiated tissue (Table 43.3) [40]. Reported rates of incontinence after surgery for such “complex” anal fistulas vary from 0 to 25 % for flatus, up to 26 % for major fecal leakage, and as high as 63 % for minor and/or passive incontinence [41]. Female gender, type of surgery, prior fistula surgery, posterior internal opening, and horizontal extension have been variables associated with postoperative incontinence. Reported fistula recurrence rates range from 0 to 30 % and have been associated with a horseshoe tract, missed tracts, failure to identify the internal opening, prior surgery, and surgeon experience [41]. For distal (low) fistulas, it is generally accepted that the risk of incontinence is minimal and fistulotomy is advocated if less than one-third of the external sphincter is crossed by the fistula [42–44].
Noncryptoglandular origin |
Cross > 30–50 % of external sphincter |
Anterior in females |
Associated with multiple tracts |
Develop in an individual with continence disturbances |
Occur in previously irradiated tissue |
Several surgical techniques have been described to address high or complex anal fistula. The anatomy of these tracts can be defined in the operating room with fistula probes and/or with the aid of dyes or hydrogen peroxide. Alternatively, radiographic evaluation with either endoanal ultrasonography (with or without hydrogen peroxide injection) (Fig. 43.3) or magnetic resonance imaging (MRI) (Fig. 43.4) may prove helpful to identify the fistula and help quantify the amount of IAS and EAS involved by the tract and at potential risk for division [45–47].
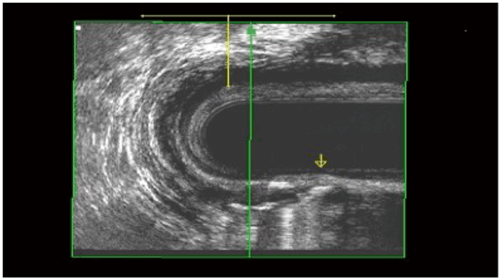
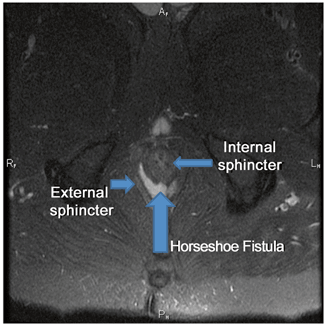

Fig. 43.3
Transsphincteric fistula with hydrogen peroxide in the tract. (Courtesy of Dr. Amy Thorsen)

Fig. 43.4
Horseshoe fistula identified on MRI. (Courtesy of Dr. Sid Walker)
Both draining and cutting setons may be used for high or complex fistulas. After defining the fistula tract with a probe, the surgeon may elect to use a combination of a seton placed through the tract and a partial sphincterotomy. A draining seton is used to assure complete resolution of associated abscesses and to induce fibrosis along the tract. When the inflammatory process has resolved, the seton may either be removed in hope that the fistula will go on to heal without further division of muscle or it can be removed and additional fistulotomy performed. Sometimes the draining seton is converted to a cutting seton or in the absence of significant associated abscess and inflammation, a cutting seton may be used instead of a draining seton in the first procedure. The cutting seton is gradually tightened to slowly divide the remaining involved muscle in the fistula tract. This theoretically allows scar to form as the seton is slowly “walked through the sphincter,” thus keeping the sphincter muscle intact and avoiding a wide gap as is noted when muscle is divided in one procedure.
Eradication of the fistula is reported to be 60–78 % with recurrence rates between 2 and 9 %. Although the cutting seton at one time was thought to preserve continence in comparison with direct division, reports have not confirmed this reputed advantage. Minor disturbance of continence occurs in 34–63 % of patients along with impaired anal manometry and postoperative deformity of the anal canal [41, 48]. Additionally, cutting setons are not well tolerated because of the discomfort associated with frequent tightening of the cutting seton. The two-stage seton fistulotomy results in similar rates of incontinence as the cutting seton with minor incontinence ranging from 54 to 66 % and major incontinence ranging from 4 to 26 % [49, 50]. Injection of fibrin glue or a collagen plug results in varying success rates ranging from 33 to 88 % with minimal associated morbidity or alteration of continence [51–53].
Endorectal advancement flaps have been used to obliterate the internal fistula opening without division of the sphincter complex in an attempt to preserve continence. Following debridement of the chronic fistula tract(s), a flap of mucosa and submucosa with or without a portion of internal sphincter muscle is mobilized beginning distal to the internal opening of the fistula. The flap is mobilized proximally increasing its width to maintain good vascularity. The proximal dissection proceeds until the mobilized flap can be advanced distally over the internal opening of the fistula and a tension-free repair of the flap to the anorectum distal to the internal opening can be achieved (Fig. 43.5). Long-term studies on advancement flaps report recurrence rates as high as 33 % in cryptoglandular disease and up to 57 % in Crohn’s associated fistula. Prior attempts at repair of the fistula have been associated with increased incontinence following advancement flaps [41]. This may be due to inadvertent sphincter injury with retractors, inelastic tissue secondary to scarring, and direct injury to the internal sphincter with mobilization. Identified key steps for successful flaps include correct identification of the fistula tract and internal opening. Sepsis must be resolved and the tract should be dry and fibrotic. Draining setons should be used liberally as a first-stage procedure to ready the operative field for an advancement flap. The external opening should be enlarged to prevent premature closure of the external opening, which could lead to a postoperative track abscess which may necessitate through the repair [54].
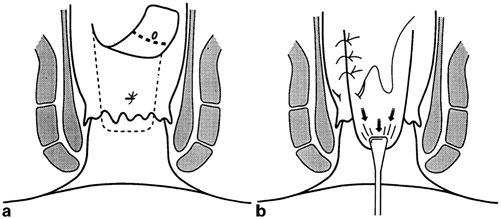

Fig. 43.5
The Ligation of the Intersphincteric Fistula Tract (LIFT) procedure is another sphincter-sparing technique that involves identification and ligation of the fistula tract in the intersphincteric groove. Success rates range from 57 to 94 % [55, 56]. Vergara-Fernandez et al. (2013) performed a review of the current LIFT literature where the primary outcomes included fistula healing rates, mean healing time, and patient satisfaction. Eighteen studies were included in the review with an N of 592. The mean healing rate was 74.6 %. Several risk factors for failure were identified and included obesity, smoking, multiple previous operations, and the long fistula tracts. Mean healing time was 5.5 weeks with a mean follow-up of 42.3 weeks. No de novo incontinence developed secondary to the LIFT procedure and patient satisfaction ranged from 72 to 100 % [57]. Currently, there is not enough evidence to assess the alleged improvement of LIFT variants.
Management
Evaluation
If anal incontinence does occur following a LIS or fistulotomy, a detailed history to assess the bowel habits including frequency of bowel movements, consistency of stools, type of incontinence (gas, liquid, solid, seepage, full bowel movement, post defecation, etc.), and severity of incontinence pre and post procedure is essential. Past history of gastrointestinal, genitourinary and neurological disorders, details of all prior anorectal procedures, medication use, and attempts to manage the incontinence should be carefully reviewed. The desire to pursue treatment of fecal incontinence depends primarily on the patient’s subjective symptoms and quality of life. A number of incontinence scales are available to help objectify these symptoms including the Cleveland Clinic Florida Fecal Incontinence (CCF-FI) scale, the Fecal Incontinence Severity Index, and the Fecal Incontinence Quality of Life Scale [58].
The physical exam should include perianal and perineal inspection looking for scars (post procedure, episiotomy), unhealed wounds, persistence of a fissure or fistula, possible prolapse (full thickness, mucosal), or signs of active infection or inflammation. Digital rectal exam is performed to evaluate possible palpable sphincter defects, assess resting tone (IAS) and squeeze (EAS). It is also important to look for use of accessory muscles (buttocks), which may be used to augment squeeze and serve as a marker for decreased function. Nerve function may be assessed by evaluating the anocutaneous reflex, which is a brief contraction of the EAS when the perianal skin is lightly stroked and indicates the presence or absence of intact sensory and motor innervation [58]. Proctosigmoidoscopy is done to exclude neoplasm, evidence of ulcerative colitis or Crohn’s disease, solitary rectal ulcer, or other disease states.
A detailed history and physical exam may provide enough information to formulate a conservative treatment plan with medical management. Frequency and stool consistency may play a significant role in the severity of the incontinence and incomplete emptying of the anorectum can result in seepage of mucus and small amounts of feces. Bulking agents and fiber supplements may play a significant role in reducing the episodes of incontinence and may be all that is necessary for those with mild incontinence. In individuals with diarrhea, it is important to investigate the cause of the diarrhea. The specific treatment should be geared toward the cause. Antidiarrheals that slow colonic transit and limit intestinal fluid secretion are beneficial for many. In a randomized, controlled trial, loperamide (Imodium) was found to be more effective than diphenoxylate-atropine (Lomotil) in patients with incontinence and may serve to increase sphincter tone [59]. An anal plug or cotton wick may be beneficial in those individuals with fecal soiling or seepage.
Biofeedback may be used if conservative management fails or in conjunction with conservative management. Biofeedback exercises may increase strength and endurance of the anal muscles and improve rectal sensation [58]. Success rates of biofeedback for incontinence range from 38 to 100 %. A sphincter defect may limit but does not preclude the possibility of a good response.
For those individuals with persisting incontinence of unclear etiology or who fail conservative management and are possible candidates for surgery, pelvic floor testing may be beneficial to evaluate pelvic floor function and anatomy. Anal manometry is used to objectively assess anal resting and squeeze pressures as well as rectal compliance. Endoanal ultrasound and MRI are useful to detect and quantify sphincter defects. Pudendal-nerve terminal motor latency (PNTML) testing allows one to quantify nerve function.
Treatment
Injectables
For patients with passive fecal incontinence (individuals with seepage or soilage secondary to IAS damage or dysfunction) and/or low resting anal pressures, intra-anal injectables have been promising. The mechanism of the injectable is to provide an increase in the resting tone to compensate for the failed IAS [58, 60]. Various materials have been injected to treat incontinence and include collagen, silicone, autologous fat, glutaraldehyde, carbon-coated beads, and dextranomer in hyaluronic acid gel [61]. The technique involves injection of the agent into the deep submucosa of the anal canal. Several studies have shown a reduction in fecal incontinence episodes with significant improvement of quality of life. However, long-term studies are lacking [61].
Magnetic Bowel Sphincter
The magnetic anal sphincter (Fenix, Torax Medical, Shoreview, MN) is currently experimental and not available for implantation outside of study. The sphincter is made of titanium beads with magnetic cores that are implanted around the anal sphincter muscle complex. In two separate cohort matched studies, the magnetic anal sphincter was comparable to the artificial bowel sphincter (ABS) or sacral nerve stimulator for improvement of fecal incontinence, quality of life, and resting anal pressures [62, 63].
Sacral Nerve Stimulator
The indications for sacral nerve stimulation (SNS) (Medtronic, Minneapolis, MN, USA) have expanded over the last decade after its introduction for fecal incontinence in 1995. Initially SNS was reserved for patients with an intact sphincter and impaired function [64]. However, its use has now evolved to include a wide spectrum of sphincter dysfunction. Randomized controlled trials have shown good long-term results with SNS. Mellgren et al. demonstrated, at 3 years follow-up, improvement of symptoms in 86 % of the 133 patients [65]. Hull et al. reported that 89 % of patients have continued reduction in fecal incontinence and 36 % had a complete response to SNS at 5 years [66]. Potential complications of the SNS include lead displacement, pain, infection, and paresthesias.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








