Study
N. of patients
Indication (%)
Mäkelä et al. [16]
106
9
Bleeker et al. [17]
213
33
Primrose et al. [18]
1202
25
Nakamoto et al. [19]
12
58
3.2.2 Positron Emission Tomography and Positron Emission Tomography/Computed Tomography
CRC is known to be FDG avid. PET/CT is an accurate modality for detecting pelvic recurrence in CRC patients and may have advantages over MDCT and MRI in differentiating scar from viable tumor (Fig. 3.1). The lack of anatomical reference hampers exact localization and evaluation of the extent of local relapse on PET alone. Since these data are essential when considering therapeutic intervention, such as re-excision or irradiation, PET/CT may be of greater value. Therefore, for detecting and evaluating LR, it is advised to perform PET/CT, when available, rather than PET alone.
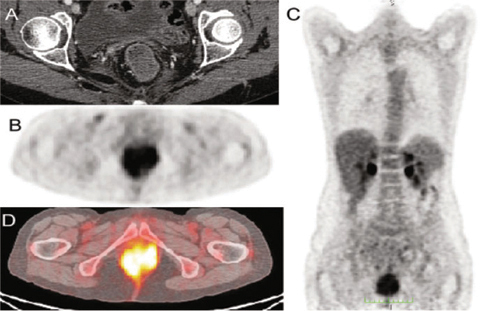

Fig. 3.1
Contrast-enhanced computed tomography (CT) (A), positron emission tomography (PET) (B, C), and PET/CT (D): colorectal cancer infiltrates the anal canal from the anastomotic region, involving the anal sphincters and levator muscles, and is not distinguishible from the posterior wall of the vagina
A recent meta-analysis [20] compared the diagnostic performance of PET/CT, CT, and MRI as whole-body imaging modalities for detecting local and/or distant recurrent disease in CRC. Patients with a high clinical and biological suspicion of recurrent disease were enrolled. Both PET and PET/CT were far more accurate than MDCT, probably mainly due to lower accuracy of MDCT for detecting extrahepatic disease, including LR.
The reported accuracy of FDG-PET for pelvic recurrences of CRC ranges from 74 to 96%, and for metastatic disease to the lung and liver, its accuracy ranges from 93 to 99% [21]. In a retrospective study, Moore et al. [22] investigated the impact of PET for detecting pelvic recurrence in 60 previously irradiated rectal cancer patients. PET imaging correctly identified 16 of 19 proven recurrences. Sensitivity, specificity, overall accuracy, positive predictive value (PPV), and negative predictive value (NPV) were 84%, 88%, 87%, 76%, and 92%, respectively. Even-Sapir et al. [23] assessed the role of PET/CT in detecting LR of rectal cancer. Sixty-two patients underwent PET/CT examination; findings were of clinical relevance in 29 of the 62 patients. PET/CT was more sensitive and specific than PET alone. Of 24 patients with pelvic recurrence, 16 had pelvic recurrence only, and eight had both pelvic and extrapelvic recurrence. Thirteen of the 24 patients were referred for surgery, and thereby, PET/CT correctly depicted 23 of the 24 pelvic recurrences. PET/CT differentiated between benign lesions from presacral recurrences with a sensitivity of 100% and a specificity of 96%. In a study from Peng and co-workers [24], FDG-PET/CT was performed on patients with elevated serum CEA levels >5 ng/mL (group 1) or on those with suspicious recurrences without rise in serum CEA levels (group 2). The authors performed 128 consecutive FDG-PET/CT analyses (49 in group 1; 79 in group 2) on 96 recruited patients; recurrences were proven in 63. Overall sensitivity, specificity, and accuracy of FDG-PET/CT were 98%, 89%, and 94%, respectively. FDG-PET/CT induced changes in planned management in 48% (62/128) of all patients. The authors concluded that FDG-PET/CT improves the survival rate of CRC patients and should be performed not only in those with elevated serum CEA levels, but also in those in whom recurrences are suspected to improve the early detection of resectable disease.
Limitations of PET/CT include suboptimal spatial resolution, poor FDG uptake in mucinous adenocarcinomas, limited specificity (false positives in patients with associated sepsis, anastomotic leakage, or postoperative inflammation), physiological uptake from organs displaced after surgery, and patients receiving chemotherapy when tumor tissue is not metabolically active [25]. Lastly, PET/CT is expensive and not always available.
3.2.3 Magnetic Resonance Imaging
MRI is known as a successful imaging modality for evaluating pelvic and rectal malignancy, particularly in the postoperative setting [26]. Although current recommendations for postoperative surveillance for LR of CRC include neither MRI nor PET/CT [27], MRI may still be necessary in selected patients with clinical, colonoscopic, and/or biochemical suspicion of recurrent disease and with equivocal or normal findings on previous imaging modalities. The anatomical information obtained with MRI, combined with the functional information provided by diffusion-weighted imaging (DWI), currently remains of value. Pelvic MRI is accurate not only for detecting pelvic recurrence CRC recurrence but also for predicting the absence of tumor invasion in pelvic structures. Thus, in may provide a preoperative road map of the recurrence to allow for appropriate surgical planning. As always, correlation of imaging and clinical findings in the multidisciplinary forum is paramount.
Distinguishing recurrent cancer within a presacral scar is more accurate when using MRI. This finding is based on differences in signal intensity between tumor and fibrosis when using T2-weighted sequences or contrast-enhanced imaging techniques [28]. Despite these advantages over other imaging tests, a recent study [2] concluded that MRI as part of routine pelvic surveillance after curative resection of CRC is not justified. Rather, MRI should be reserved for selectively imaging patients with clinical, colonoscopic, and/or biochemical suspicion of recurrent disease. The study examined 226 patients who underwent curative surgery for CRC. An intensive follow-up program included clinical examination, CEA measurement, colonoscopy, and MRI at 3- to 6-month intervals. The separate contribution of each diagnostic test to the final diagnosis was assessed. The median clinical follow-up was 42 months, with a median MRI surveillance period of 21 months and a median number of MRI scans per patient of three. LR was detected in 13% of the patients. The median interval between initial surgery and recurrence was 15 months. MRI detected 87% of LR but missed three of the four anastomotic recurrences. In summary, sensitivity, specificity, PPV, and NPV were 87%, 86%, 48%, and 98%, respectively. MRI was the only positive diagnostic test in four (13%) patients with pelvic recurrence located in the perirectal tissue, with two of these patients deemed to have resectable disease. Resection of local relapse was possible in 20% of patients. MRI correctly diagnosed four of these six cases. The median survival time in the surgically treated group was 13 months and in the unresectable group (24) 9 months. In light of these results, the authors strongly questioned the use of MRI in routine postoperative follow-up of CRC patients: 576 examinations were performed in 226 patients in order to detect only four cases with LR missed by other tests.
MR examination is based on T2-weighted (T2-w) fast spin-echo (FSE) sequences acquired in the sagittal, axial, and coronal planes using a 1.5- or 3-Tesla scanner. Gadolinium-enhanced FSE T1-w sequences may additionally be performed, as well as diffusion-weighted imaging (DWI). No preparation or rectal distension is necessary.
3.2.4 Contrast-Enhanced and Dynamic Contrast-Enhanced Magnetic Resonance Imaging
Conventional contrast-enhanced MRI (CE-MRI) examinations are limited in their role in differentiating postoperative or postradiation therapy changes from LR. Benign fibrotic scarring, malignant local tumor recurrence, and inflammation can all enhance after the administration of a gadolinium-based contrast agent [29]. A longer interval between radiation therapy or surgery and imaging improves the accuracy of MRI in identifying fibrosis, since fibrosis tends to mature and develop low T2 signal over time [30]. The coexistence of a tumor with significant fibrosis causing low signal intensity on T2-w images may be the main reason for the “undercalling” of recurrence at MRI [30, 31].
In addition to the use of T2 signal to differentiate tumor from fibrosis, shape and enhancement patterns may help distinguishing these two entities. Tumors tend to have round borders, whereas fibrosis has straight angular margins. Additionally, tumors tend to have contrast enhancement >40% of the volume of a mass or a typical rim-enhancement pattern after gadolinium injection [30, 31].
Dynamic contrast-enhanced MRI (DCE-MRI) could be useful to differentiate benign fibrotic scar from LR using the time-intensity curve (TIC) obtained following the contrast agent kinetic after gadolinium injection on a region of interest (ROI) or volume of interest (VOI) segmented by the radiologist [32–36]. The typical TIC shows a low enhancement in case of fibrotic scar and a rapid and strong enhancement in case of LR (Figs. 3.2 and 3.3).
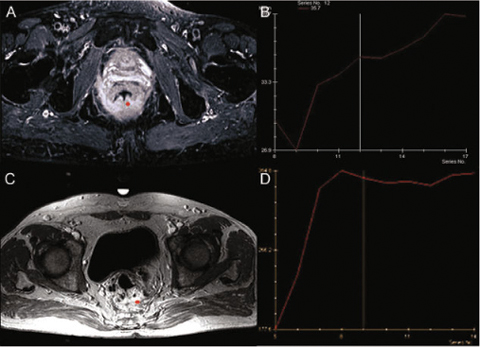
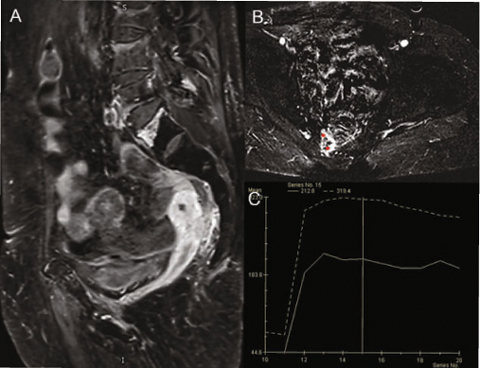

Fig. 3.2
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) differentiating benign fibrotic scar (A, B) from local recurrence (C, D)

Fig. 3.3
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI): sagittal image (A), axial image (B), with time-intensity curve (TIC) visual inspection (C); presacral colorectal cancer recurrence
3.2.5 Diffusion-Weighted Magnetic Resonance Imaging
The role of MRI and DWI-MRI has been evaluated in a recent retrospective study [37]. The authors reported a high accuracy in the diagnosis of locally recurring rectal cancer when recurrence is suspected. In a series of 42 patients suspected of having local rectal recurrence and including 19 recurrences, the authors demonstrated that DWI does not significantly improve the diagnostic performance. However, they observed a trend toward an additional value of DWI in improving the specificity for recurrence diagnosis. Indeed, a relevant limitation of MRI generally is represented by overestimation of tumor presence within areas of postoperative scarring tissue [38, 39]. DWI clearly discriminates the abnormal signal intensity of tumor from fibrosis and from surrounding organs, such bowel.
Moreover, the benefit of DWI might be more obvious in detecting small anastomotic recurrences (Figs. 3.4–3.5).
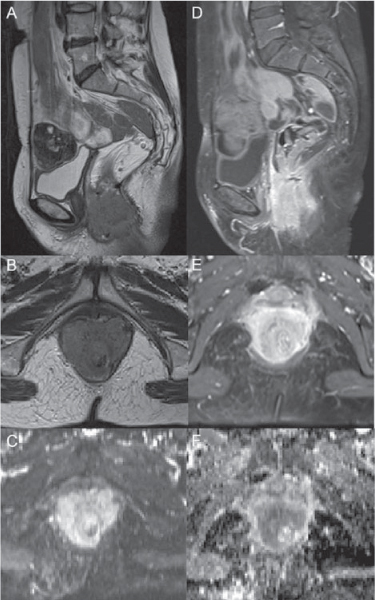
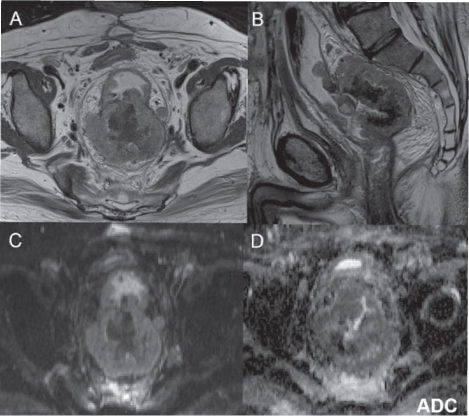

Fig. 3.4
T2-weighted magnetic resonance imaging (MRI) (A, B), contrastenhanced MRI (CE-MRI) (D, E), diffusion-weighted MRI (DW-MRI) at b800 (C), and apparent diffusion coefficient (ADC) map (F): colorectal cancer that infiltrates the anal canal from the anastomotic region, with involvement of anal sphincters and levators is not dissociable from the posterior vaginal wall

Fig. 3.5
Magnetic resonance imaging (MRI) (A, B), diffusion-weighted MRI (DW-MRI) at b800 (C), and apparent diffusion coefficient (ADC) map (D): colorectal cancer recurrence corresponding with the anastomosis
3.3 Systematic Literature Review
Which imaging modality is the most accurate for assessing LR of CRC? To answer this question, we performed a systematic review of the literature, evaluating studies that estimated diagnostic performance, such as sensitivity, specificity, PPV, NPV, and accuracy. In Table 3.2, we summarize the findings for all individual studies.
Table 3.2
Diagnostic performance for all included studies
Study | Modality | N. of patients | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
[13] | CT | 83 | 88 | 98 | 94 | 94 | 96 |
[14] | CT | 18 | 82 | 50 | 69 | 67 | 68 |
[40] | CT | 15 | 75 | 100 | 100 | 78 | 87 |
[41] | CT | 40 | 73 | 75 | 88 | 53 | 74 |
[42] | CT | 45 | 68 | 50 | 86 | 25 | 64 |
[43] | CT | 47 | 71 | 70
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|

