Histologic Features
General Structure
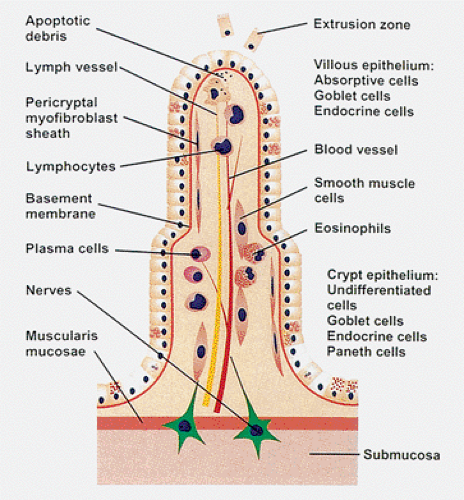
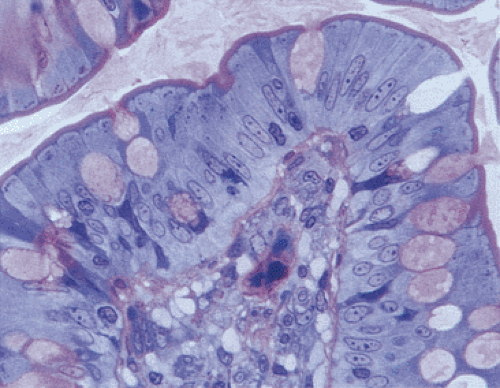
Small intestinal epithelium is organized into two morphologic and functionally distinct compartments: The crypts of Lieberkühn and the villi. Villi that are unique to the adult small intestine are fingerlike or leaflike mucosal evaginations lined by epithelium overlying a connective tissue core that contains a highly cellular lamina propria, a capillary network, lacteals, and nerves. Simple tubular invaginations (crypts of Lieberkühn) at the base of the villi extend down toward the muscularis mucosae but do not penetrate it (Figs. 6.20 and 6.21). Several crypts open into the intervillous basin.
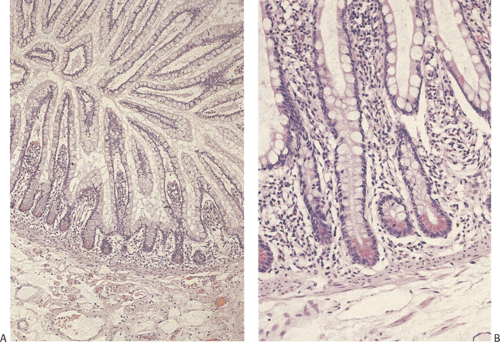
Villi vary in height and form in different regions of the small bowel. The duodenum has the greatest villous variability. Villi in the proximal duodenum are shorter and broader than elsewhere, not infrequently showing increased numbers of stunted and leaf-shaped or branched forms when compared to the jejunum. Jejunal villi vary little in their width from their base to their apex. In the ileum, the villi become broader and shorter than in the jejunum (Fig. 6.21). Villous morphology also varies among different ethnic groups and geographic locales. Villi in persons from Thailand, Africa, India, South Vietnam, and Haiti are shorter and thicker and have an increased portion of leaf-shaped forms with a more intensely cellular lamina propria when compared to biopsies from the English or Northern Americans (40). It is unclear whether this variation represents true racial differences or results from exposure to the infections endemic to the former areas.
The ratio of villous height to crypt length, a feature best appreciated in well-oriented sections, allows one to assess
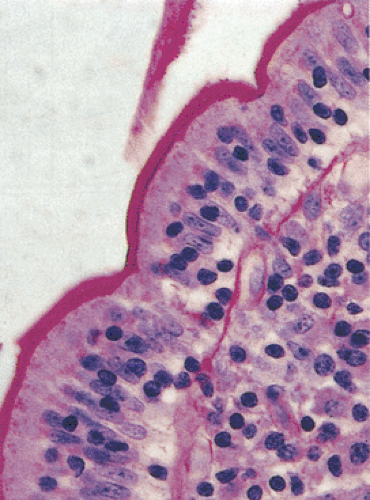
small intestinal absorptive function. In adults, villous height is approximately three or more times the length of the crypts, whereas in children this ratio is lower, more typically being 2:1. Villous height is also lower in the elderly (41). The duodenal crypt:villus ratio is 3:1 to 7:1, whereas the ileal crypt:villus ratio is 4:1. Villi overlying lymphoid areas are often stubby or absent. Each villus contains an arteriole with capillary network veins and a central lymphatic as well as numerous nerve fibers (Fig. 6.22).
small intestinal absorptive function. In adults, villous height is approximately three or more times the length of the crypts, whereas in children this ratio is lower, more typically being 2:1. Villous height is also lower in the elderly (41). The duodenal crypt:villus ratio is 3:1 to 7:1, whereas the ileal crypt:villus ratio is 4:1. Villi overlying lymphoid areas are often stubby or absent. Each villus contains an arteriole with capillary network veins and a central lymphatic as well as numerous nerve fibers (Fig. 6.22).
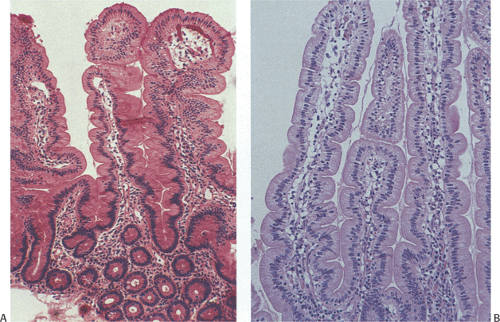 FIG. 6.21. A comparison of proximal ileal (A) versus jejunal (B) villi. The more distal villi are more irregular in shape than those of the proximal region. |
Each crypt consists of a single clone of cells; several crypts contribute cells to each villus. The epithelial lining harbors a heterogeneous cell population, including Paneth cells, undifferentiated crypt cells, endocrine cells, cup cells, tuft cells, goblet cells, absorptive cells (enterocytes), and M cells. Each cell type possesses distinctive structural features and functions (see below). The epithelium maintains a close association with the underlying stroma.
Cell Proliferation and Differentiation
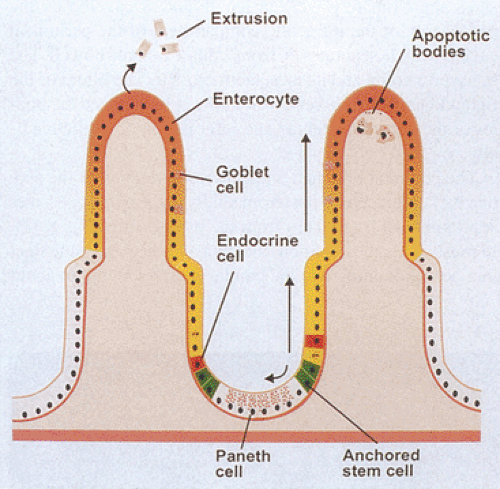
Maintaining the integrity of the gut epithelium as well as ensuring its continuous turnover is essential for mucosal defense. As a result, the gut has one of the most rapid proliferative rates in the body (42). Regular cellular renewal maintains an equilibrium between cell birth and cell death. When the mucosa is damaged, replacement of the injured cells guarantees mucosal integrity. New epithelial cells arise from a fixed proliferating stem cell population located in the lower part of the crypt (Figs. 6.23 and 6.24) (43,44,45). These pluripotential stem cells give rise to descendants that undergo three or four divisions while migrating up the villus or to the top of the lymphoid dome (44).
The duration of cellular proliferation and migration is approximately 5 to 6 days in most of the human small intestine and 3 days in the ileum. Differentiated enterocytes live for little more than 2 days (46). Cells at the villous tip undergo Fas-mediated apoptosis (47) and slough off and are extruded into the lumen; sloughing cells can also be seen on the edges of the villi (48). Apoptotic cell death occurs without any apparent disruption of the mucosal barrier integrity (49).
Mitotic figures are present in the deep crypt, but they are never normally present on the villi. Stem cells give rise to four major epithelial cell types: Absorptive cells (enterocytes), goblet cells, endocrine cells, and Paneth cells (44). The production and maturation of these cells is under the control of homeobox genes including MATH1, Cdx1, and Cdx2 (3,17,18) and the Wnt signaling pathway (45). The MYC-MAD-MAX network is another key regulator of intestinal cell maturation (45).
Newly produced cells migrate out of the crypt, differentiate, and assume the functional characteristics of mature surface cells. Precursors of absorptive enterocytes comprise 90%
of the cells in the crypt and mature absorptive cells comprise 95% of the cells located on proximal intestinal villi (43,44). The other three primary epithelial phenotypes constitute a small but important percentage of the total cell number. Cell migration occurs in a linear fashion, with cells moving directly vertically upward or downward from their site of genesis in the crypt bases. The process of proliferation and cellular differentiation are topologically well organized and maintained within this progressively differentiating epithelium. As the cells mature, enterocytes gain digestive enzymes. Gene expression profiling studies show that different genes are differentially expressed in the crypt and on the villi. Genes that are up-regulated in the crypt and down-regulated at the villous tip include those related to the cell cycle, RNA processing, and protein translation. In contrast, genes related to cytoskeletal assembly, lipid uptake, and enzyme biosynthesis show the opposite pattern (50).
of the cells in the crypt and mature absorptive cells comprise 95% of the cells located on proximal intestinal villi (43,44). The other three primary epithelial phenotypes constitute a small but important percentage of the total cell number. Cell migration occurs in a linear fashion, with cells moving directly vertically upward or downward from their site of genesis in the crypt bases. The process of proliferation and cellular differentiation are topologically well organized and maintained within this progressively differentiating epithelium. As the cells mature, enterocytes gain digestive enzymes. Gene expression profiling studies show that different genes are differentially expressed in the crypt and on the villi. Genes that are up-regulated in the crypt and down-regulated at the villous tip include those related to the cell cycle, RNA processing, and protein translation. In contrast, genes related to cytoskeletal assembly, lipid uptake, and enzyme biosynthesis show the opposite pattern (50).
There are also specific specializations of intestinal cells, such as the M cell, which derive from the multipotential stem cell. M cells play a major role in antigen sampling from the GI mucosa and are described in a later section.
Enterocytes (Absorptive Cells)
Enterocytes are highly polarized cells with two structurally and functionally distinct plasma membrane domains: The apical microvillous membrane and the basolateral membrane. The apical domain includes the brush border and extends to the tight junction that forms a band around the membrane, creating a relatively impermeable joint between adjacent epithelial cells. The remainder of the cell membrane constitutes the basolateral domain. The basolateral membranes contain abundant Na+, K+-ATPase and adenylate cyclase and are the site of the receptor for dimeric IgA attachment before its transport to the apical membrane. It is also the transfer site for chylomicrons and other foodstuffs from the enterocyte into the intercellular space and the lamina propria (Fig. 6.25). This activity is restricted from the apical surface by tight junctions that maintain these differences and prevent lateral movement of membrane components (51).
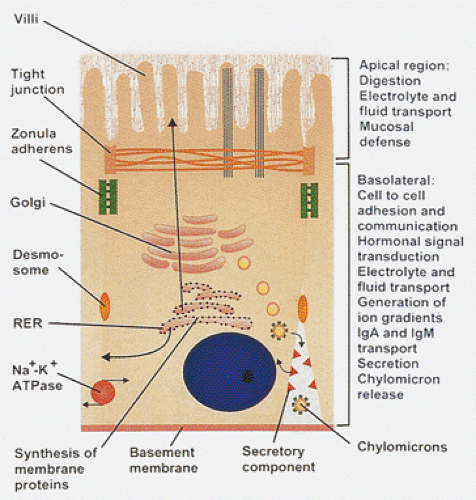 FIG. 6.25. Enterocytes are highly polarized cells with distinct apical, lateral, basolateral, and basal portions, each of which serves special roles. |
Enterocytes continuously synthesize new components of the cell membrane and surface coat and transport them to the microvillous surface. Microvilli exhibit bidirectional cell trafficking with various metabolites being absorbed and transported inward while the hydrolytic enzymes are synthesized in the endoplasmic reticulum, glycosylated in the Golgi, and transported to the brush border for insertion into the brush border membranes (Fig. 6.25). Some intestinal diseases result from impaired membrane protein trafficking including microvillous inclusion disease, congenital sucrase-isomaltase deficiency, and adult lactase deficiency disease.
The mature brush border, which covers the cell apex, consists of closely packed microvilli and the terminal web (Fig. 6.26). Microvilli vary in length, increasing in height as the cells migrate up the crypt–villus axis. Mature microvilli
measure approximately 1.5 to 2 μm in length and 100 nm in diameter. These structures are periodic acid–Schiff (PAS) positive (Fig. 6.27). Each microvillus contains a core bundle of approximately 20 vertically oriented, polarized actin filaments extending from the tip of the microvillus to the base of the terminal web (Fig. 6.28). These are cross-linked by the actin-bundling proteins fimbrin and villin. The other major actin-binding protein of the microvillous core is myosin 1 (52). Myosin 1, coupled with calmodulin, forms a double spiral of bridges cross-linking the actin bundles to the plasma membrane (53). The microvilli house a wide array of brush border enzymes that play critical roles in the digestion and absorption of proteins, fats, and carbohydrates. A complex anastomosing meshwork of filaments called the terminal web surrounds the microvillous rootlets. It consists of a network of actin filaments cross-linked with myosin 2, nonerythroid spectrins, α-actinin, and tropomyosin (52). The filamentous network links with the junctional complex at the edge of the cell.
measure approximately 1.5 to 2 μm in length and 100 nm in diameter. These structures are periodic acid–Schiff (PAS) positive (Fig. 6.27). Each microvillus contains a core bundle of approximately 20 vertically oriented, polarized actin filaments extending from the tip of the microvillus to the base of the terminal web (Fig. 6.28). These are cross-linked by the actin-bundling proteins fimbrin and villin. The other major actin-binding protein of the microvillous core is myosin 1 (52). Myosin 1, coupled with calmodulin, forms a double spiral of bridges cross-linking the actin bundles to the plasma membrane (53). The microvilli house a wide array of brush border enzymes that play critical roles in the digestion and absorption of proteins, fats, and carbohydrates. A complex anastomosing meshwork of filaments called the terminal web surrounds the microvillous rootlets. It consists of a network of actin filaments cross-linked with myosin 2, nonerythroid spectrins, α-actinin, and tropomyosin (52). The filamentous network links with the junctional complex at the edge of the cell.
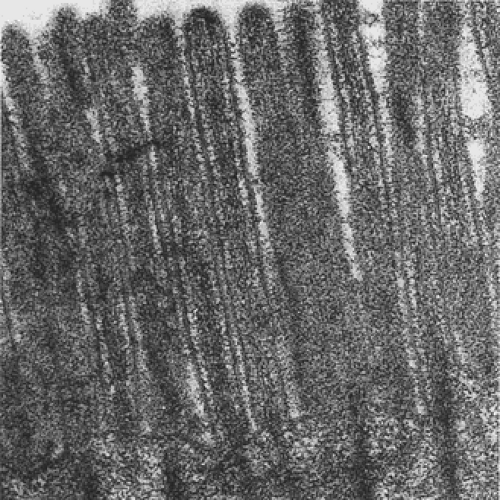 FIG. 6.28. Normal small intestine. Striated border of absorptive cells is made of large numbers of closely packed parallel microvilli. |
The intercellular space is a dynamic area. When the cell is in a resting state, it remains collapsed and represents a potential space. In contrast, the intercellular space dilates in actively transporting cells, particularly at its most basal part (54). The junctional complex, a series of intercellular junctions, is present at the apical end of the intercellular space. The most basal member of this complex is usually the desmosome, a macular structure resembling a spot weld or adhesion point between adjacent epithelial cells (55). The zonula adherens (ZA), or intermediate junction, is a more apically located circumferential adhesive structure. Filaments from the ZA extend into the terminal web to form part of the cytoskeleton. The tight junction or zonula occludens lies at the most apical aspect of the lateral cell surface and it surrounds each epithelial cell, forming a gasketlike seal that restricts the movement of substances through the paracellular pathway by forming semipermeable barriers (56). Signaling via interactions of the cytoskeleton with the tight junctions may regulate paracellular permeability of solutes and water. Diverse microfilament-associated proteins contribute to the cellular morphology, motility, and other cellular specialized functions.
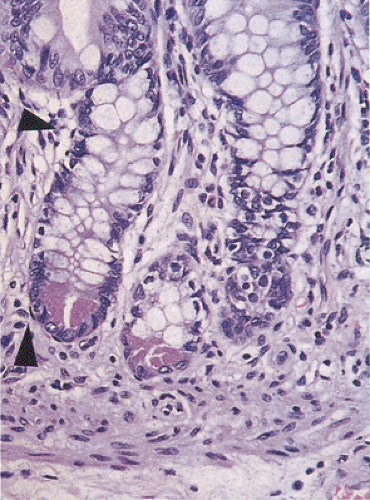 FIG. 6.29. Pericryptal fibroblasts are flattened fusiform cells closely apposed to the crypt basement membrane (arrows). Notice the Paneth cells in the crypt base identified by their eosinophilic apical granules.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|