Prostate cancer (CaP) is the second most common cause of cancer deaths in the United States and the incidence of CaP has remained constant at 165 cases per 100,000 men. Since 1990, the age-adjusted death rate has decreased by 31%. In this article, the authors review the current literature on the experimental therapy for HIFU. The HIFU technique, its mechanism of action, patient selection, current efficacy studies, complications, follow-up after HIFU treatment, and future developments are discussed.
Prostate cancer (CaP) is the second most common cause of cancer deaths in the United States, and the incidence of CaP has remained constant at 165 cases per 100,000 men. Since 1990, the age-adjusted death rate has decreased by 31%. The decrease in death rate is most likely due to early detection and treatment. The decrease in mortality has not been as significant as expected compared with the increase in diagnosis of CaP. The discrepancy between incidence and mortality has been attributed to increasing detection of clinically insignificant tumors.
Furthermore, prostate screening (ie, digital rectal examination [DRE] and prostate specific antigen [PSA]) may identify clinically insignificant cancers and result in over diagnosis and over treatment of prostate cancer. CaP screening may result in over treatment of prostate cancer by at least 30%. Since the beginning of the PSA era, CaP screening has shifted the disease burden to organ-confined and lower-grade disease. Etzioni and colleagues estimated that 10% of men with low-grade prostate cancer are over treated with radical surgery, and 45% are over treated with radiation therapy.
Some of the side effects of radical prostatectomy and radiation therapy include urinary incontinence and impotence. The incidence of these morbidities has decreased with improved technique; however, these morbidities are significant for individuals who may be over treated for their otherwise indolent CaP.
Once diagnosed with CaP, a patient must make an informed decision on which mode of treatment to pursue. This decision is made more difficult by the varied modalities, invasiveness, outcomes, and return to baseline function after treatment. Moreover, treatment type depends on clinical stage, Gleason grade, patient preference, and other comorbid conditions. Treatment types include active surveillance, radical prostatectomy, cryotherapy, and radiation therapy (either brachytherapy or external-beam radiation). In addition, several new and innovative therapies such as high-intensity ultrasound (HIFU) are being studied.
HIFU was introduced 15 years ago for the treatment of benign prostatic hypertrophy. In 1996, Gelet and colleagues used HIFU for the treatment of localized low-grade CaP. Many studies have been performed to evaluate the use of HIFU for low-grade, localized prostate cancer. HIFU has also been used as salvage therapy after radiation. The National Institute for Clinical Excellence (NICE) in the UK evaluated HIFU in 2005 and found that there was sufficient evidence to recommend its use for the treatment of CaP. However, in 2008, NICE only recommended the use of HIFU in controlled clinical trials or when patients are entered into a registry and closely followed. The French Association of Urology (FAU) and the Association of Italian Urologists (AURO) now recommend HIFU as standard treatment for patients with localized disease, who are unsuitable for or who failed radiation, or who are unsuitable for surgery. The European Association of Urology guidelines, however, state HIFU is “investigational or experimental.” In the United States, HIFU is currently not approved for treatment of CaP outside ongoing investigational trials. As more studies elucidate long-term disease-free rates, it is expected that consensus recommendations on the use of HIFU for localized CaP will soon emerge.
Recent response to the over diagnosis of CaP and over treatment of CaP by urologists has led to the need to consider other forms of therapy that have less morbid side effects and are less invasive. HIFU is a minimally invasive treatment of CaP and needs to be evaluated for efficacy that is similar to or exceeds other modalities of treatment, minimum side effects, quicker recovery from treatment, and hopefully reduced treatment costs. HIFU uses ultrasound energy to cause mechanical and thermal injury to the target tissue. In this article, the authors review the current literature on the experimental therapy for HIFU. The HIFU technique, its mechanism of action, patient selection, current efficacy studies, complications, follow-up after HIFU treatment, and future developments are discussed.
HIFU technique
HIFU, when used for the treatment of localized prostate cancer, uses an ultrasound transducer placed in the rectum to generate acoustic energy that is focused on the tissue target, creating high temperatures and irreversible coagulative necrosis. HIFU uses a trackless principle, whereby tissue outside the focal plane is not damaged; the transrectal probe sits on the rectal mucosa and sends acoustic energy through the intervening tissues, only heating the tissue volume targeted by the probe. The probe is repositioned mechanically as needed to target the entire prostate. This technique is minimally invasive, requires less anesthesia and involves a shorter recovery period than surgery, and can be performed in a day surgery setting.
HIFU is generally performed with the patient under spinal or general anesthesia. The operation can last from 1 to 4 hours, and should not be performed with prostate volumes greater than 40 mL. Often, a limited transurethral resection of the prostate (TURP) is performed before application of HIFU to reduce the risk of postoperative urinary retention. (Notably, study protocols of US trials do not permit the use of TURP before HIFU.) The patient is placed in the lithotomy position. The ultrasound probe is covered with a condom and inserted gently into the rectum using lubricating jelly. Once inserted, an articulating arm aids in maintaining the position of the probe. Cool water (17–18°C) is circulated through the condom to protect adjacent tissues from thermal damage throughout the procedure. The prostate is visualized using real-time diagnostic images generated by the probe using lower, nondestructive acoustic energies (0.1–100 mW/cm 2 ). Once the target areas are identified, the prostate tissue is ablated with high energies (1300–2200 W/cm 2 ) focused in a small 1- to 3-mm-wide by 5- to 26-mm-long focal plane. Each pulse heats the tissue to 80 to 98°C over a 3-second period. The gland is revisualized with lower ultrasound energies between ablative pulses. The probe is then moved and rotated in a semi-automated manner (device-dependent) using lower-energy diagnostic images to target adjacent prostate tissue. The end goal is to create overlapping lesions until the whole gland is treated. Patients often require a urethral or suprapubic catheter for several days.
Body movement and breathing pose continued challenges to the application of this technology. In addition, the small target volumes make it more difficult to achieve homogeneous treatment of the entire gland. Because the HIFU device settings are based on animal models with presumed uniform tissue characteristics, further difficulty arises from uneven absorption of the acoustic energy influenced by possible heat-resistant tumor cells, prostatic calcifications, and differences in local blood perfusion.
Current market products
Two commercially-available ultrasound-guided transrectal devices are currently used for the treatment of prostate cancer: the Ablatherm (EDAP TMS, Lyon, France) and the Sonablate 500 (Focus Surgery, IN, USA). While these devices are approved in the treatment of localized prostate cancer in Asia and Europe, their use in the United States is currently limited to investigational, phase III trials only. Both HIFU devices are trackless, in that no tissue is damaged between the probe and the targeted area of tissue in the focal plane. The original Ablatherm employs two probes each with piezoceramic transducers, has fixed-power settings, and requires a transurethral resection of the prostate (TURP) to be performed preoperatively due to limitations in depth of treatment. A newer version uses a single probe with two transducers. One transducer is dedicated to imaging and the other to treatment, enabling real-time visualization during treatment. Additionally, the newer Ablatherm device has three dedicated treatment parameters for the different clinical scenarios, including primary treatment, repeat HIFU, and salavage therapy. The Sonablate 500 device offers greater mobility and customization of treatment settings, allowing for more surgeon control over the HIFU beam characteristics, including adjustment of focal length, energy and power delivered to the target. The device enables users to tailor treatment to the particular characteristics of a patient’s prostate and disease burden through transverse and sagittal low-energy, real-time imaging and software monitoring of tissue changes. The Sonablate 500 also incorporates imaging of blood flow around the neurovascular bundles; a clinician can alter treatment based on this visual feedback. Because the volume of tissue targeted by the Sonablate 500 is smaller than that of the more automated Ablatherm device, more manual manipulation of the transrectal probe is required.
Current market products
Two commercially-available ultrasound-guided transrectal devices are currently used for the treatment of prostate cancer: the Ablatherm (EDAP TMS, Lyon, France) and the Sonablate 500 (Focus Surgery, IN, USA). While these devices are approved in the treatment of localized prostate cancer in Asia and Europe, their use in the United States is currently limited to investigational, phase III trials only. Both HIFU devices are trackless, in that no tissue is damaged between the probe and the targeted area of tissue in the focal plane. The original Ablatherm employs two probes each with piezoceramic transducers, has fixed-power settings, and requires a transurethral resection of the prostate (TURP) to be performed preoperatively due to limitations in depth of treatment. A newer version uses a single probe with two transducers. One transducer is dedicated to imaging and the other to treatment, enabling real-time visualization during treatment. Additionally, the newer Ablatherm device has three dedicated treatment parameters for the different clinical scenarios, including primary treatment, repeat HIFU, and salavage therapy. The Sonablate 500 device offers greater mobility and customization of treatment settings, allowing for more surgeon control over the HIFU beam characteristics, including adjustment of focal length, energy and power delivered to the target. The device enables users to tailor treatment to the particular characteristics of a patient’s prostate and disease burden through transverse and sagittal low-energy, real-time imaging and software monitoring of tissue changes. The Sonablate 500 also incorporates imaging of blood flow around the neurovascular bundles; a clinician can alter treatment based on this visual feedback. Because the volume of tissue targeted by the Sonablate 500 is smaller than that of the more automated Ablatherm device, more manual manipulation of the transrectal probe is required.
Mechanism of action
The application of high-intensity ultrasound in medicine began with studies in 1954 by Lindstrom and Fry, who were investigating the possibility of its use in the treatment of neurologic disorders. Fry and colleagues also discovered that in focusing these high-energy acoustic waves, they could be used safely in vivo. Attempts were made to apply its use in tumors of various organs throughout the 1970s, but at lower energies for long durations. Without any method to measure target tissue temperatures noninvasively, however, this investigational treatment modality fell out of favor. In the 1980s, extracorporeal shockwave lithotripsy came to the forefront with its approval for use by the Food and Drug Administration (FDA) in 1984, allowing for the noninvasive treatment of kidney stones. In the 1990s, HIFU as a treatment of soft-tissue tumors was revived after advancements in the underlying technology, namely with the introduction of noninvasive tissue temperature monitoring. Its origination in the treatment of prostate cancer came from the canine prostate experiments by Gelet, Bihrle, and Kincaide and colleagues.
HIFU destroys target tissue through the thermal and mechanical effects of nonionizing, acoustic radiation (ie, sound waves) delivered to target tissues after focusing by an acoustic lens, bowl-shaped transducer, or electronic phased array ( Fig. 1 ). Because HIFU uses nonionizing radiation, it can be repeated one or more times in multiple sessions. The thermal effects are achieved by heating tissues to 60°C or higher, resulting in near-instantaneous coagulative necrosis and cell death. By focusing the energy, more destruction occurs within the focal plane, but tissues outside the target area are spared damage, as energy intensities are far lower.
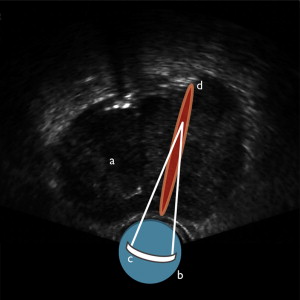
Mechanical Effects of HIFU
The use of high-frequency sound waves results in various, significant mechanical effects on the tissues in addition to the thermal effects just mentioned. These include cavitation, microstreaming, and radiation forces. Cavitation is the creation or movement of gas in an acoustic field. As the tissue compresses and expands with exposure to the acoustic waves, gas is extracted creating bubbles. These bubbles interact with the acoustic field and begin to oscillate violently. The bubbles collapse and create high-velocity jets that disrupt cell membranes. Microstreaming refers to the rapid movement of liquid outside an oscillating bubble generated through cavitation forces. When the bubbles oscillate, nearby tissues are subjected to shearing forces that can also disrupt cell membranes. Radiation forces are the pressures tissues endure when either absorbing or reflecting sound waves. Because tissues and solids respond differently from liquid media, movement of liquids can create streaming and shearing effects that disrupt cell membrane integrity. Overall, the primary mechanism of cell death in HIFU therapy is coagulative necrosis, but the sum contribution of thermal and mechanical effects is ultimately responsible for ablation of target tissues.
It has been hypothesized that these mechanical effects might contribute to local spread of tumor cells, limiting the clinical efficacy of HIFU. Several studies have refuted this claim in vitro and in vivo.
Other Effects
The high temperatures also induce the creation and release of chemically reactive free radicals. These have direct and indirect activity on surviving cells, namely in the induction of apoptosis and activity on nuclear DNA. Nearby tissues are also believed to undergo apoptosis induced by the lower levels of acoustic radiation and heat experienced during HIFU treatment. Necrosis and cavitation take days to months to peak and are believed to correlate with PSA nadir.
Limitations
Despite being a noninvasive modality in the treatment of prostate cancer, it is not without any untoward side effects. Because HIFU works best in contiguous tissues, its use is limited to localized prostate cancer; it is not meant to treat disseminated, widespread, or otherwise inoperable cancers. In addition, because ultrasound is the basis of HIFU, unwanted effects of diagnostic ultrasound imaging also apply to its higher-energy use: shadowing and refraction. Shadowing can result from large prostatic calcifications (>10 mm in diameter), which can interfere with the delivery of acoustic energy. This could potentially impact the ability to completely ablate larger glands greater than 40 mL, limiting clinical efficacy. Reflection of sound waves into nearby tissues outside the focal plane, although normally of no consequence in diagnostic imaging, could produce burns in tissues adjacent to the treatment zone (rectum, bowel, bladder).
Clinical Use of HIFU
Much of the literature focuses on the use of HIFU in the setting of primary treatment of clinically localized prostate cancer (T1c–T2a). The minimally invasive characteristics of HIFU also make it suitable as a salvage treatment option for patients with biochemical failure after other types of primary treatment, namely after radical prostatectomy or external-beam radiotherapy. HIFU does not preclude the use of other future treatment modalities; that is, prostatectomy and radiotherapy have been safely performed following HIFU treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




