CHAPTER 80 Hepatitis E
Hepatitis E is a form of acute, icteric, self-limited viral hepatitis caused by the hepatitis E virus (HEV). The disease was first recognized in the 1980s, when sera collected during the first recorded epidemic in Delhi, India, in 1955,1 and during another epidemic in Kashmir, India, in 1978,2 were found to lack serologic markers of hepatitis A and B.3 In retrospect, several epidemics of enterically transmitted hepatitis with epidemiologic features resembling those of hepatitis E outbreaks occurred in Europe in the 18th and 19th centuries. HEV was identified in 1983 by immune electron microscopy4; its genome was cloned in 1990 and fully sequenced shortly thereafter.5,6
VIROLOGY
HEV is a small RNA virus, 32 to 34 nm in diameter, nonenveloped, and icosahedral. HEV RNA is approximately 7.2 kilobases in length, single- and positive-stranded, 5′-capped, and polyadenylated. The crystal structure of HEV-like particles that consist of capsid protein has been found to exhibit the protruding domain that is involved in the binding of HEV to susceptible cells and that contains some neutralization epitopes.7 Other investigators have found that each capsid protein contains 3 linear domains that form distinct structural elements: the continuous capsid (S), 3-fold protrusions (P1), and 2-fold spikes (P2) and have speculated that binding of the susceptible cell receptor to P1 may result in conformational changes that eventually lead to cell membrane penetration and genome release into the infected cell.8
The virus is currently classified in a separate genus named Hepevirus in the family Hepeviridae.9 The HEV genome contains three open reading frames (ORFs) (Fig. 80-1).6 ORF1 encodes nonstructural proteins, ORF2 encodes the viral capsid protein, and ORF3 encodes a protein of unknown function. Details of HEV replication in hepatocytes and its release from infected cells remain unknown; the application of the replicon system to HEV research may generate a better understanding of the intracellular events during virus replication.10 Phylogenetic analysis of HEV isolates shows the existence of four geographically distinct genotypes, termed genotypes 1 to 4 (Table 80-1).11 Genotype 1 includes various isolates from Asia that have a nucleotide sequence homology of 92% to 99% (amino acid sequence homology 95% to 99%) with each other. These isolates have nucleotide homology of 75% (amino acid homology 86%) with genotype 2 isolates, which include a strain from Mexico and some isolates from western Africa. Isolates from the United States, classified as genotype 3, are 92% identical to each other but only 73.5% to 74.5% identical to genotypes 1 and 2.12 Genotype 3 isolates have also been reported from human cases in several European countries (including the United Kingdom, France, the Netherlands, and Spain), Japan, and South America. Genotype 4 consists of isolates from some parts of China, Taiwan, Japan, and Vietnam. All HEV genotypes belong to a single serotype because they share at least one major serologically cross-reactive epitope.
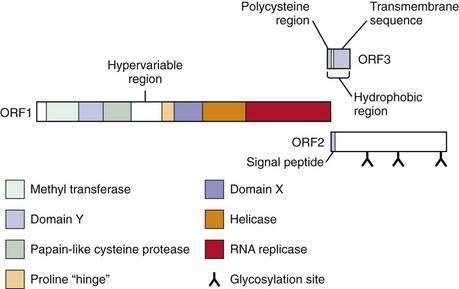
Figure 80-1. The genome of hepatitis E virus. The three open reading frames—ORF1, ORF2, ORF3—are shown.
Table 80-1 Hepatitis E Virus Genotypes and Their Geographic Distribution
| GENOTYPE | HUMAN CASES | ANIMALS |
|---|---|---|
| 1 | — | |
| 2 | Mexico, Western Africa | — |
| 3 | USA, South America, Europe (France, Spain, UK, the Netherlands), Japan | |
| 4 | China, Taiwan, Japan, Vietnam | India, China |
Adapted from Schlauder GG, Frider B, Sookoian S, et al. Identification of 2 novel isolates of hepatitis E virus in Argentina. J Infect Dis 2000; 182:294-7.
Swine HEV was identified in pigs in the midwestern United States13; the virus naturally infects pigs and induces transient viremia and antibodies that react with human HEV strains. A comparison of swine HEV with the Burmese and Mexican isolates of human HEV showed approximately 90% to 92% and 79% to 83% identity at the amino acid level in the ORF2 and ORF3 regions, respectively. HEV-like genomic sequences have been isolated from pigs in several parts of the world and occasionally from other animals, including deer and wild boar. In geographical regions where genotype 3 and 4 HEV has been reported among humans, swine isolates of HEV have also belonged to these genotypes. Also, swine HEV strains in Taiwan and Spain have greater genetic similarity to human HEV strains from these regions than to swine and human HEV isolates from other parts of the world, suggesting the possibility of zoonotic transmission. By contrast, swine HEV identified in India is genetically different from the HEV in patients from the same geographic region.14,15 An HEV-like virus has been recovered from chickens and its genome partially sequenced; it appears to be noninfectious to mammals.16
EPIDEMIOLOGY
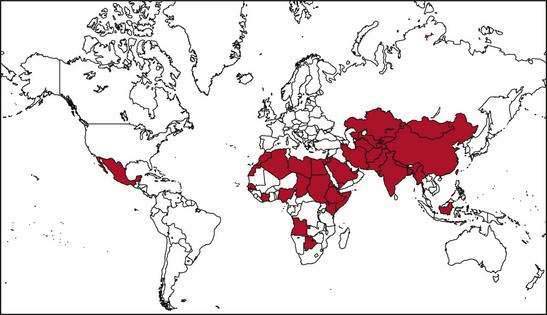
Several epidemics of hepatitis E affecting several hundred to several thousand persons have occurred on the Indian subcontinent and in southeast and central Asia, where this infection is endemic.1,2,17–19 Outbreaks of hepatitis E also have been reported from the northern and western parts of Africa and the Middle East. Two small outbreaks were reported in Mexico in 1986 and 1987 (Fig. 80-2). Overall attack rates range from 1% to 15% and are higher for adults (3% to 30%) than for children (0.2% to 10%). The male-to-female ratio among cases has ranged from 1:1 to 4:1, but the outbreaks have been characterized by particularly high attack and mortality rates among pregnant women. The epidemics vary in nature, ranging from single-peaked, short-lived outbreaks to prolonged, multipeaked epidemics lasting more than a year (Table 80-2). In endemic areas, hepatitis E accounts for up to 50% to 70% of cases of sporadic acute hepatitis; these cases are demographically and clinically similar to those observed during disease outbreaks.
Table 80-2 Epidemiologic Features of Hepatitis E
In nonendemic regions, hepatitis E is related mostly to travel to HEV-endemic regions and accounts for fewer than 1% of cases of acute viral hepatitis. Solitary cases or small series of cases with indigenously-acquired cases of acute hepatitis E have been reported from several countries in Europe, North America, and Australia. A report from the United Kingdom described 40 patients with hepatitis E and no history of travel to an area endemic for this disease. HEV infection was thought to be acquired locally, and the patients were described as a group of “autochthonous” hepatitis E cases.20 Although most patients found to have HEV infection in this series had jaundice, a few had anicteric illness with nonspecific symptoms or asymptomatic aminotransferase elevations. The number of cases peaked in spring and summer, and disease appeared to be more common in residents of coastal and estuarine areas. The disease showed seasonal variations with peaks in spring and summer and no cases occurring during November and December. Case series with similar characteristics have been described from southwest France and the Netherlands.21,22 Table 80-3 highlights differences in the epidemiologic and clinical features of HEV genotypes 1 and 3.
Table 80-3 Comparison of Epidemiologic and Clinical Features Associated with HEV Genotypes 1 and 3
| HEV GENOTYPE 1 | HEV GENOTYPE 3 | |
|---|---|---|
| Epidemiologic patterns | Large epidemics, small outbreaks, and sporadic cases | Only sporadic cases |
| Animal-to-human transmission | Not reported | Demonstrated; a likely mode of transmission |
| Water-borne transmission | Well known to occur; most common route | Unknown |
| Animal reservoir | No | Yes |
| Age group | Young men most commonly affected | Usually elderly |
| Chronic infection | Not known to occur | Reported in transplant recipients receiving immunosuppressive drugs |
| Severity | Variable severity, including fulminant hepatic failure | Severity and poor outcome are related to comorbid conditions |
ROUTES OF TRANSMISSION
HEV infection is transmitted predominantly through the fecal-oral route. Most reported outbreaks have been related to consumption of fecally contaminated drinking water (see Table 80-2). The outbreaks frequently follow heavy rains and floods, but some epidemics occur during the hot summer months, when decreased flow in rivers may increase the risk of water contamination. Person-to-person transmission of hepatitis E seems to be uncommon during both epidemic and sporadic settings,23,24 and secondary attack rates among household contacts are only 0.7% to 2.2%. Therefore, recurrent epidemics in endemic regions are probably related to nearly continuous fecal contamination of water. This conclusion is supported by the demonstration of HEV RNA in waste water, sewage, and drinking water in endemic regions25 and of viable HEV (infectious to primates) in occasional sewage specimens in nonendemic regions.26
Mechanisms postulated to contribute to contamination of water sources by HEV and maintenance of the virus in endemic areas include the occurrence of subclinical HEV infection, animal reservoirs that harbor HEV, and prolonged fecal shedding of the virus by humans. Although data on subclinical HEV infection in humans in endemic regions are scant, viral excretion has been demonstrated in an experimental macaque model of subclinical HEV infection.27 Proven incidents of animal-to-human transmission of HEV have been shown to occur in Japan, where hepatitis E developed after ingestion of uncooked deer meat; the viral genomic sequences obtained from these cases were similar to those retrieved from left-over meat.28 Genomic sequences from human cases of hepatitis E in the United Kingdom have resembled those from swine isolates of HEV, both belonging to genotype 3. HEV genomic sequences were isolated from pig livers sold in grocery stores in Japan and in the United States and were closely related to those from human cases of hepatitis E in those countries.29,30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree