Chronic hepatitis C virus (HCV) infection is a major public health burden in Europe, being one of the leading causes of chronic liver disease, liver cirrhosis, and hepatocellular carcinoma. Properties of the HCV disease burden are heterogeneous across the European continent, with differences in incidence, prevalence, diagnosis and treatment rates, transmission routes, and genotype distribution. Recent estimates expect an increase in HCV-related morbidity and mortality in most European countries until 2030 even when current treatment options are taken into account. The European perspective on hepatitis C virus infection is summarized herein.
Key points
- •
Properties of the hepatitis C virus (HCV) disease burden are heterogeneous across Europe with differences in incidence, prevalence, diagnosis and treatment rates, transmission routes, and genotype distribution.
- •
Injective drug use has replaced medical procedures as the major transmission risk factor for HCV in Europe.
- •
Recent estimates expect an increase in HCV-related morbidity and mortality in most European countries until 2030 even when current treatment options are taken into account.
- •
Highly efficient interferon-free treatment regimens are available for all HCV genotypes. Interferon-containing treatment regimens have limited relevance in some European countries with less developed health care systems and for selected difficult-to-cure patients.
- •
Accessible and affordable treatment options with high efficacy have to be complemented by improved prevention and screening strategies for a sustained reduction of HCV-related morbidity and mortality on the European continent.
Introduction
Chronic HCV infection is a major public health burden in Europe, being one of the leading causes of chronic liver disease, liver cirrhosis, and hepatocellular carcinoma (HCC). As a consequence, chronic HCV infection is a common cause for liver transplants across the continent. The prevalence of HCV infection in the geographic area of Europe ranges from 0.4% to 1.5% in Western Europe and 0.7% to 3.2% in Central Europe to 0.9% to 1.7% in Eastern Europe. The limited availability of reliable data from some regions, especially Eastern Europe, and the fact that some patient populations such as prison inmates or socially excluded groups with a higher HCV prevalence are not reached by surveys lead to an underestimation of the total number of infected individuals. Owing to the diverse historical developments and backgrounds in different European countries and regions, the characteristics of the HCV-infected population and risk factors for transmission are highly variable across European countries. To date, injective drug use is the leading cause for chronic HCV infection in most parts of Europe, whereas contaminated syringes in medical procedures were a major cause of infection in rural areas with limited medical care in Eastern European countries such as Greece, Turkey, and Romania. A substantial proportion of the HCV-infected population in most Western European countries, such as Belgium, France, and Germany, acquired the infection in the 1970s and 1980s similar to the high proportion of baby boomers among HCV-infected patients in North America, whereas the increase in injective drug use (IDU) in many Eastern European countries during the last 2 decades led to more recent HCV infection and a younger HCV-infected cohort in countries such as the Czech Republic. That is why epidemiologic models predict the peak prevalence of HCV-related complications (transplant, cirrhosis, and HCC) by 2030 for Western Europe and much later for parts of Eastern Europe.
HCV treatment is still changing rapidly as the first approved direct antiviral agents (DAAs) telaprevir and boceprevir were outpaced by new highly effective and interferon-free treatment regimens within the last 2 years. In Europe, NS5B polymerase inhibitor sofosbuvir, NS3/4A protease inhibitor simeprevir, and NS5A inhibitor daclatasvir in interferon-based and interferon-free treatment and combination regimens, as well as the ritonavir-boosted combination of NS3/4A protease inhibitor paritaprevir and NS5A inhibitor ombitasvir with NS5B-polymerase inhibitor dasabuvir, are approved by the European Medicines Agency for the treatment of chronic HCV infection according to genotype, prior treatment, and stage of liver disease. Nevertheless, interferon-containing treatment regimens with or without the first-generation NS3/4A protease inhibitors telaprevir and boceprevir are still in use in some Eastern European countries with less developed health care systems owing to the lower treatment costs.
The European perspective on HCV infection regarding epidemiologic features of disease burden in European countries, consequences of chronic HCV-related liver disease, and future challenges, as well as the currently available treatment options, are reviewed herein.
Introduction
Chronic HCV infection is a major public health burden in Europe, being one of the leading causes of chronic liver disease, liver cirrhosis, and hepatocellular carcinoma (HCC). As a consequence, chronic HCV infection is a common cause for liver transplants across the continent. The prevalence of HCV infection in the geographic area of Europe ranges from 0.4% to 1.5% in Western Europe and 0.7% to 3.2% in Central Europe to 0.9% to 1.7% in Eastern Europe. The limited availability of reliable data from some regions, especially Eastern Europe, and the fact that some patient populations such as prison inmates or socially excluded groups with a higher HCV prevalence are not reached by surveys lead to an underestimation of the total number of infected individuals. Owing to the diverse historical developments and backgrounds in different European countries and regions, the characteristics of the HCV-infected population and risk factors for transmission are highly variable across European countries. To date, injective drug use is the leading cause for chronic HCV infection in most parts of Europe, whereas contaminated syringes in medical procedures were a major cause of infection in rural areas with limited medical care in Eastern European countries such as Greece, Turkey, and Romania. A substantial proportion of the HCV-infected population in most Western European countries, such as Belgium, France, and Germany, acquired the infection in the 1970s and 1980s similar to the high proportion of baby boomers among HCV-infected patients in North America, whereas the increase in injective drug use (IDU) in many Eastern European countries during the last 2 decades led to more recent HCV infection and a younger HCV-infected cohort in countries such as the Czech Republic. That is why epidemiologic models predict the peak prevalence of HCV-related complications (transplant, cirrhosis, and HCC) by 2030 for Western Europe and much later for parts of Eastern Europe.
HCV treatment is still changing rapidly as the first approved direct antiviral agents (DAAs) telaprevir and boceprevir were outpaced by new highly effective and interferon-free treatment regimens within the last 2 years. In Europe, NS5B polymerase inhibitor sofosbuvir, NS3/4A protease inhibitor simeprevir, and NS5A inhibitor daclatasvir in interferon-based and interferon-free treatment and combination regimens, as well as the ritonavir-boosted combination of NS3/4A protease inhibitor paritaprevir and NS5A inhibitor ombitasvir with NS5B-polymerase inhibitor dasabuvir, are approved by the European Medicines Agency for the treatment of chronic HCV infection according to genotype, prior treatment, and stage of liver disease. Nevertheless, interferon-containing treatment regimens with or without the first-generation NS3/4A protease inhibitors telaprevir and boceprevir are still in use in some Eastern European countries with less developed health care systems owing to the lower treatment costs.
The European perspective on HCV infection regarding epidemiologic features of disease burden in European countries, consequences of chronic HCV-related liver disease, and future challenges, as well as the currently available treatment options, are reviewed herein.
The epidemiology of hepatitis C virus infection in Europe
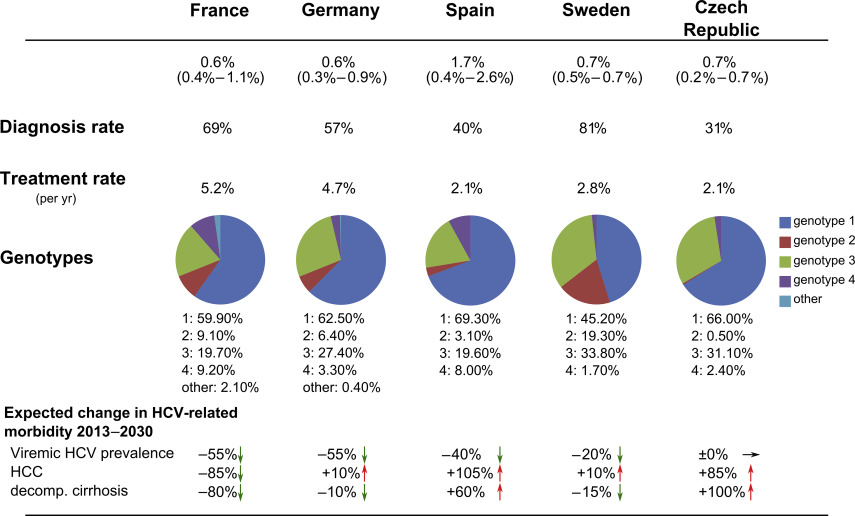
Epidemiologic data on HCV prevalence, diagnosis rate, genotype distribution, and major risk factors for HCV transmission for several representative European countries are illustrated in Fig. 1 .
Hepatitis C Virus Prevalence, Diagnosis Rate, and Screening Strategies
For interpretation of the HCV prevalence in Europe, it is worth knowing that the epidemiologic data are based mostly on reports from national HCV surveillance systems, which leads to a heterogeneity in data quality and completeness between countries. Especially in some Eastern European countries where no HCV surveillance system exists, the data were extrapolated from local studies. Furthermore, selected groups with a naturally high HCV prevalence, such as prison inmates or socially excluded groups, are not represented sufficiently in the reports, leading to an underestimation of the total number of infected individuals.
The prevalence of HCV infection in the geographic area of Europe ranges from 0.4% to 1.5% in Western Europe and 0.7% to 3.2% in Central Europe to 0.9% to 1.7% in Eastern Europe. HCV hotspots with a prevalence of 3% or more are found in Romania and some rural areas in Greece and Italy. Thus, estimations for the total number of HCV-infected persons in Europe range from 11,500,000 to 19,000,000. As highly efficient treatment options become available, the new diagnosis of to date unknown HCV infections is a key factor and a prerequisite for the efficient reduction of the European HCV disease burden. The diagnosis rate varies between European countries and is high in Denmark (59%), France (69%), Germany (57%), and Sweden (81%). Low diagnosis rates are found in the Czech Republic (31%), Portugal (33%), and Turkey (16%). HCV screening programs are implemented in almost all European countries for organ and blood donors as well as for patients undergoing hemodialysis, but up to now risk groups such as injecting drug users, prison inmates, or health care workers are not included in routine HCV screenings in some parts of Europe. In 2012, an HCV screening program for all baby boomers born between 1945 and 1965 was implemented in the United States because more than 75% of HCV-infected patients belong to that age group. In Europe, similar programs were considered but not yet implemented as the age distribution is not as narrow as in the United States. The growing challenge of HCV infections in migrants is not yet addressed by screening programs, although this sensitive subject deserves attention as, for example, approximately one-quarter of HCV-infected patients in Germany are not of German origin.
Effective screening programs for risk groups proved to be successful and cost-effective. The access to HCV screening and subsequent treatment of injecting drug users, who form the new major HCV cohort in Europe for upcoming decades, is an elementary task on the European continent.
Routes of Hepatitis C Virus Transmission
Iatrogenic transmission
Before screening assays were available, blood transfusions or invasive medical procedures were the leading cause for HCV transmission in the 1970s and 1980s across Europe. After the implementation of routine screening assays in 1990, the infection rates by blood transfusion were reduced tremendously from 0.45% to less than 0.001% per blood unit. Simultaneously, the high rate of HCV transmission in hemophiliacs by contaminated clotting factors was eliminated by the use of recombinant products. According to a French cross-sectional survey, HCV transmission in hemodialysis units was reduced to 0.45% per year and the prevalence of anti-HCV antibodies in patients undergoing dialysis has decreased in most European countries. Although the transmission of HCV by invasive medical procedures was reduced significantly in most Central and Western European countries over the last decades, hospital admissions and invasive medical procedures were still among the major risk factors in 2 studies among patients with acute hepatitis C infection in the 1990s and 2000s in Spain and Germany. The extraordinary high prevalence in some areas of southern Italy is due to the frequent use of glass multiuse syringes for intravenous therapies in the past. A continued high iatrogenic transmission rate in Europe is observed in rural areas with limited medical care, such as in parts of Romania, Crete, or Turkey. Nevertheless, IDU has outpaced other risk factors of HCV transmission in most European countries, and individuals with IDU will form the future major HCV cohort on the continent.
Injective drug use
Most newly acquired HCV infections in Europe are related to injective drug use. Up to 1 million active injective drug users live in the European Union with a prevalence of anti-HCV antibodies ranging between 45% and 90%. The incidence of HCV infection was highest in the 1980s and early 1990s before the implementation of HIV prevention programs. Prevention strategies against IDU-transmitted infections by enhanced access to sterile injection equipment, self-injection facilities, professional counseling, and substitution programs have lowered transmission rates among illicit drug users in Western Europe during the last decades. According to the Swiss HIV cohort study, the HCV incidence in individuals with IDU decreased from 13.89 per 100 person-years in 1998 to 2.24 in 2011. Similar progress was reported for other Western European countries. However, the infection rates among injective drug users even in Western Europe remain high, and further measurements to improve prevention, diagnosis rates, and treatment access are necessary. Prevention programs are implemented insufficiently or lacking in most Eastern European countries, including Russia leading to an increase in HCV incidence during the last years parallel to the increase in IDU.
Hepatitis C virus in the migrant population
An increasing proportion of the HCV-infected cohort in Western Europe consists of immigrants coming from countries with high HCV prevalence. Approximately a quarter of HCV-infected patients in Germany and The Netherlands are migrants mostly from Poland, Russia, and Turkey. In all European countries (with reliable data) except Italy, the anti-HCV prevalence was shown to be higher in the migrant population than in the general population of the same country. Hence, the implementation of screening strategies for migrants from high-prevalence countries might help to reduce HCV disease burden and long-term complications in the future.
Genotype Distribution
HCV genotype 1 accounts for most HCV infections across the European continent (Western Europe, 59%; Eastern Europe, 65%; Central Europe, 89%) followed by genotype 3 (25%, 30%, 9%), genotype 2 (10.8%, 4.4%, 0.1%), and genotype 4 (4.9%, 0.1%, 1.3%). The genotype distribution is influenced considerably by routes of HCV transmission. Transmission by contaminated blood products was traditionally related to infections with genotype 1b, whereas genotypes 1a and 3 are associated with IDU-related infections. Accordingly, in countries with IDU as the major transmission route in the prevalent HCV cohort (eg, United Kingdom, Sweden), genotypes 1a and 3 account for most cases. Countries where most infections are associated with iatrogenic transmission (eg, Spain, Turkey), genotype 1b is most common. As IDU outpaced transmission by blood products or medical procedures as the main risk factor across the European continent, genotypes 1a and 3 are on the increase, especially in countries with an increasing number of illicit drug users, mostly in Eastern Europe. Genotype 4 is more common in Central and Southern Europe (Spain 8%, France 9.2%, Switzerland 10.3%, Greece 13.9%) in contrast to North America, where it accounts for less than 2% of HCV infections. That is why pharmaceutical companies promoted the approval of their therapeutic regimens with genotype 4 activity for the treatment of genotype 4 in the European Union, whereas approval by the US Food and Drug Administration (FDA) for genotype 4 is lacking.
Hepatitis C Virus–Related Mortality, Transplant, and Future Perspectives
Hepatitis C virus and mortality
HCV infection is one of the leading causes of chronic liver disease in Europe. After 20 to 30 years of chronic HCV infection, 20% to 30% of the patients have developed liver cirrhosis with a risk to develop HCC of 1% to 4% per year. The World Health Organization (WHO) mortality reports include data on deaths associated with acute HCV infection, liver cirrhosis, and HCC. It is estimated that about 35% of deaths related to cirrhosis and 32% related to HCC are associated with HCV infection in the WHO region of Europe. Based on those numbers, in 2012, about 96,000 deaths in Europe can be attributed to HCV infection leading to an HCV-related mortality of 10.61 per 100,000, which is higher than the mortality related to human immunodeficiency virus infection/AIDS (92,700; 10.2 per 100,000). The mortality associated with HCV-related HCC follows an East-West gradient with a higher mortality observed in Western Europe. The opposite effect applies for the mortality of HCV-related cirrhosis, which is higher in Eastern Europe. These findings might be related to a higher prevalence of coexisting risk factors in HCV-infected patients in Eastern Europe, such as excessive alcohol intake or coinfection with hepatitis B, leading to an increase in mortality in patients with cirrhosis before they develop HCC. Furthermore, the diagnosis and reporting of HCCs might be less reliable in parts of Eastern Europe with less developed health care systems.
Hepatitis C virus and transplant
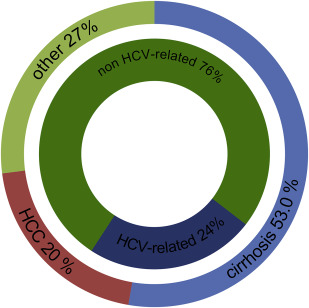
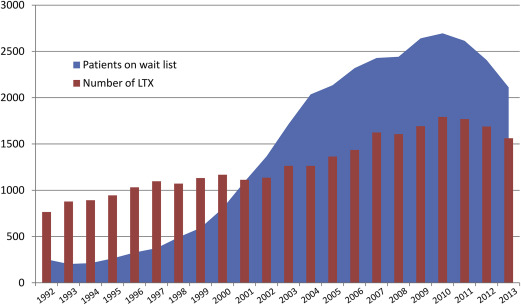
HCV is one of the leading causes for liver transplant in Europe. Prospective data on all liver transplants in Europe are collected by the European Liver Transplant Registry (ELTR). About 11,454 of 48,218 (24%) liver transplants between 1999 and 2009 were HCV related (assuming that 32% of HCC-associated transplants were HCV related as no HCV-specific data for HCC is published) ( Fig. 2 ). In the same period, HCV infection accounted for 60% of transplants with viral hepatitis-related cirrhosis as the primary diagnosis. Given the high proportion of patients with HCV infection on the liver transplant waiting list and the mismatch of needed and available organs, it is obvious that a substantial amount of waiting list mortality concerns patients with HCV. The disparity of the number of waiting list patients and organ transplants in the Eurotransplant region (Austria, Belgium, Croatia, Germany, Hungary, Luxembourg, The Netherlands, and Slovenia) emerging for more than a decade is illustrated in Fig. 3 . Recent estimates expect the number of liver transplants for HCV-related liver disease to increase until the year 2030 in almost all European countries even when current treatment options are taken into account. Accordingly, future efforts have to focus on HCV prevention to further reduce HCV incidence as well as on the improvement of diagnosis and treatment rates to reduce the HCV disease burden in Europe for the upcoming decades.
Future perspectives of hepatitis C virus disease burden in Europe
The incidence of HCV infection is decreasing across Europe because of the decrease in iatrogenic HCV transmissions and the implementation of prevention programs against IDU-transmitted infections. In addition, with the beginning of the current millennium and the introduction of pegylated interferon, effective treatment possibilities that helped to decrease the existing HCV disease burden became available. This development will be boosted enormously by the currently available treatment options with DAAs. Nevertheless, although a study that modeled the future developments of HCV disease burden in Europe predicted a decrease in viremic HCV infections in most European countries (Belgium, Denmark, England, France, Germany, Portugal, Spain, Sweden, Switzerland, and Turkey) except the Czech Republic until 2030, an increase in the number of HCCs, in compensated and decompensated cirrhosis, and in liver-related mortality is expected in the same period. This development is associated with the aging of the large number of patients who acquired the infection more than 20 years ago, forming a cohort with a peak prevalence of HCV-related complications to be expected in the next 15 years. As diagnosis and treatment rates are historically high in Austria, France, Germany, and Sweden, it is estimated that the prevalence of HCV-related sequelae will peak in those countries before 2030. In countries such as the Czech republic, with a young HCV cohort that acquired the infection more recently, mostly by IDU, complications of chronic HCV infection are estimated to peak even after 2030. The change in the HCV-related morbidity in representative European countries is illustrated in Fig. 1 . These data emphasize that efficient treatment options alone are insufficient in the struggle for a reduction of HCV-related complications in the European population. Rather, a team play of improved prevention and screening strategies with accessible and affordable treatment options is essential for a sustained reduction of HCV-related morbidity and mortality.
Approved hepatitis C virus treatment options in the European Union
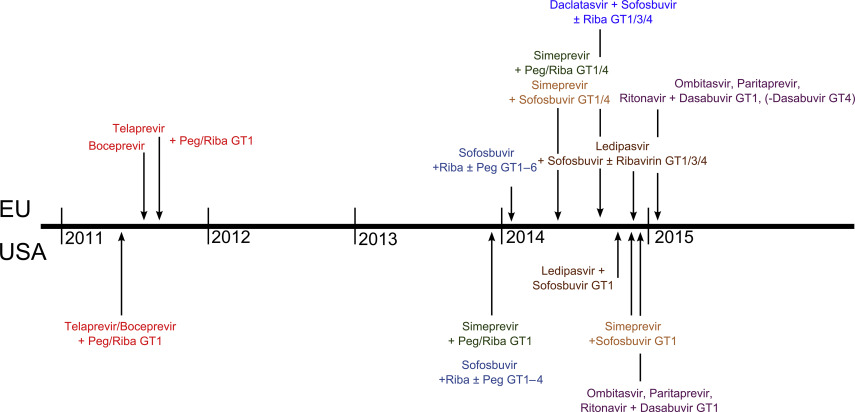
Since the approval of the first DAAs telaprevir and boceprevir in 2011, the portfolio of highly effective HCV treatment options has increased tremendously. In the last 2 years, 7 new DAAs have been approved in the European Union, which are NS5B polymerase inhibitor sofosbuvir, NS3/4A protease inhibitor simeprevir, and NS5A inhibitor daclatasvir, as well as the ritonavir-boosted combination of NS3/4A protease inhibitor paritaprevir and NS5A inhibitor ombitasvir with NS5B polymerase inhibitor dasabuvir. Fig. 4 illustrates the timeline for DAA approvals in the United States and Europe, showing the dense order of approvals within the last year. Most therapeutic regimens were approved in close succession in the United States and Europe; only for daclatasvir FDA approval is still pending. With the availability of highly effective and short interferon-free treatment protocols even for former difficult-to-cure patients with cirrhosis or therapy experience, interferon-based therapies can no longer be recommended as first-line treatments. Nevertheless, interferon keeps its relevance in countries with less developed health care systems and limited access to the new DAAs as well as for selected difficult-to-cure cases. The following paragraphs summarize the available interferon-free treatment options for the different genotypes and their implementation in European treatment protocols. Owing to the density of new DAA approvals in the last year, some treatment options are not yet implemented into guidelines.