Chronic infection with the hepatitis C virus (HCV) is a major cause of liver disease worldwide and is also responsible for extrahepatic manifestations (EHMs) involving the skin, kidneys, salivary glands, eyes, thyroid, and immune system. Mixed cryoglobulinemia is the prototype EHM related to HCV infection. Although these HCV-related EHMs may contribute to significant rates of morbidity affecting patient’s quality of life and survival, most of these complications can reverse after HCV eradication by interferon therapy. This notwithstanding, individual patients may have an irreversible injury in various organs that is not reversed by a cure of the HCV infection.
Key points
- •
Hepatitis C virus (HCV) infection is associated with injury of organs other than the liver, which is thought to contribute to increased rates of morbidity and all-cause mortality.
- •
Extrahepatic manifestations (EHMs) of HCV infection are variegate because they include mixed cryoglobulinemia (MC), lymphomas, membranous glomerulonephritis, porphyria cutanea tarda (PCT), lichen planus, thyroiditis, sicca syndrome, polyarthritis, diabetes mellitus (DM), cardiovascular diseases, and neurocognitive impairment.
- •
MC is the dominant EHM because it can be detected in half of all HCV-infected patients, yet less than 5% of the affected subjects develop a cryoglobulinemic syndrome.
- •
HCV eradication through antiviral therapy protects against the clinical consequences of such EHMs as cryoglobulinemic vasculitis, glomerulonephritis and polyneuropathy, lymphoma, and diabetes. Deferral of HCV infection treatment favors the onset of irreversible organ injury.
Introduction
HCV infection is a multifaceted disease, associated with chronic and typically slowly developing injury of the liver and a potential to affect other organs including the kidney, skin, thyroid, eyes, joints, nervous system, and immune system. In the past decades, a variety of symptoms occurring in HCV-infected patients such as fatigue, musculoskeletal pain, depression, and irritability have been recognized and attributed to EHMs of HCV infection. More recently, both in the West and in the East, the role of HCV in all-cause mortality has also emerged through studies comparing the outcome of untreated patients with patients treated with antiviral therapy. Although cirrhosis-related death invariably is the dominant cause of mortality in all studies, significant rates of deaths caused by extrahepatic events have also been identified. As a matter of fact, HCV has been recognized as the major viral cause of MC, which in turn affects half of all chronically infected patients and may be implicated in multiorgan damage. Some cases of MC have been associated with other viral infections, including human immunodeficiency virus, Epstein Barr virus, and parvoviruses. Evidence has also accumulated supporting an association between HCV infection and an increased risk of lymphoproliferative disorders in the domain of B-cell non–Hodgkin lymphoma (NHL), type 2 diabetes, cerebrovascular and cardiovascular events, PCT, and lichen planus. It is not surprising, therefore, that the lifetime cumulative risk for an HCV-infected patient to develop at least one EHM is thought to largely exceed 50%, likely being a consequence of either the ability of the virus to target and infect lymphocytes or the virus expressing reactive proteins, which boost tissue deposition of immune complexes and initiation of immune-mediated cytotoxic reactions. While EHMs such as MC have strongly been associated with HCV both clinically and pathologically, the link between the virus and other clinical complications is difficult to explain, being mostly supported by prevalence studies and, in a few reports, by additional response to antiviral treatment ( Box 1 ). The latter is the case for the basis of an association between chronic HCV infection and B-cell NHL, which relies on robust epidemiologic data coupled with reports of prevention and reversal of the tumor after HCV eradication with antiviral therapy. More recently, the central nervous system (CNS) involvement of HCV infection resulting in impairment of several neurocognitive functions has been substantiated by clinical studies coupled with brain imaging investigations, leading to the demonstration that such a dysfunction was reversible on treatment-related clearance of HCV.
Established association
MC/cryoglobulinemic vasculitis
B-cell NHL
Significant association
PCT
Lichen planus
Monoclonal gammophaties
Possible association
Sicca syndrome
Corneal ulcers (Mooren ulcers)
Thyroid disease
Noncryoglobulin nephropathies
Neuropathy
Pulmonary fibrosis
Type 2 diabetes
Arthralgias, myalgias, inflammatory polyarthritis
Autoimmune thrombocytopenia
Introduction
HCV infection is a multifaceted disease, associated with chronic and typically slowly developing injury of the liver and a potential to affect other organs including the kidney, skin, thyroid, eyes, joints, nervous system, and immune system. In the past decades, a variety of symptoms occurring in HCV-infected patients such as fatigue, musculoskeletal pain, depression, and irritability have been recognized and attributed to EHMs of HCV infection. More recently, both in the West and in the East, the role of HCV in all-cause mortality has also emerged through studies comparing the outcome of untreated patients with patients treated with antiviral therapy. Although cirrhosis-related death invariably is the dominant cause of mortality in all studies, significant rates of deaths caused by extrahepatic events have also been identified. As a matter of fact, HCV has been recognized as the major viral cause of MC, which in turn affects half of all chronically infected patients and may be implicated in multiorgan damage. Some cases of MC have been associated with other viral infections, including human immunodeficiency virus, Epstein Barr virus, and parvoviruses. Evidence has also accumulated supporting an association between HCV infection and an increased risk of lymphoproliferative disorders in the domain of B-cell non–Hodgkin lymphoma (NHL), type 2 diabetes, cerebrovascular and cardiovascular events, PCT, and lichen planus. It is not surprising, therefore, that the lifetime cumulative risk for an HCV-infected patient to develop at least one EHM is thought to largely exceed 50%, likely being a consequence of either the ability of the virus to target and infect lymphocytes or the virus expressing reactive proteins, which boost tissue deposition of immune complexes and initiation of immune-mediated cytotoxic reactions. While EHMs such as MC have strongly been associated with HCV both clinically and pathologically, the link between the virus and other clinical complications is difficult to explain, being mostly supported by prevalence studies and, in a few reports, by additional response to antiviral treatment ( Box 1 ). The latter is the case for the basis of an association between chronic HCV infection and B-cell NHL, which relies on robust epidemiologic data coupled with reports of prevention and reversal of the tumor after HCV eradication with antiviral therapy. More recently, the central nervous system (CNS) involvement of HCV infection resulting in impairment of several neurocognitive functions has been substantiated by clinical studies coupled with brain imaging investigations, leading to the demonstration that such a dysfunction was reversible on treatment-related clearance of HCV.
Established association
MC/cryoglobulinemic vasculitis
B-cell NHL
Significant association
PCT
Lichen planus
Monoclonal gammophaties
Possible association
Sicca syndrome
Corneal ulcers (Mooren ulcers)
Thyroid disease
Noncryoglobulin nephropathies
Neuropathy
Pulmonary fibrosis
Type 2 diabetes
Arthralgias, myalgias, inflammatory polyarthritis
Autoimmune thrombocytopenia
Mixed cryoglobulinemia
MC is an autoimmune, lymphoproliferative disorder characterized by circulating immune complexes named cryoglobulins (CGs) that reversibly precipitate at low temperatures. MC is the dominant and most thoroughly documented EHM of HCV infection, which in some patients results in systemic vasculitis after the deposition of CGs in small- and medium-sized blood vessels. The relationship between HCV and CGs is substantiated by the exceedingly high rates (up to 90%) of patients with CGs who have circulating anti-HCV antibodies and the finding that about half of all HCV-infected patients have circulating CGs, although less than 5% of these subjects will ultimately develop an overt MC syndrome (MCS). As the prevalence of MC increases with the duration of the infection, not surprisingly, liver abnormalities were found to persist almost twice as long in patients with MC as in those without it. Although clinically benign, MC is classified as a lymphoproliferative disorder that predisposes to NHL in about 5% to 10% of the patients at a risk threshold that is about 35 times higher than that in the general population. CGs consist of polyclonal IgGs and monoclonal or polyclonal IgM with rheumatoid factor activity sustained by the clonal expansion of B cells and are categorized on the basis of the clonality of the responsible immunoglobulin. In type I CGs, the cryoprecipitate contains an isolated monoclonal immunoglobulin IgG or IgM, whereas type II CGs are mixed, with a cryoprecipitate consisting of polyclonal immunoglobulins (mainly IgG) mixed with monoclonal immunoglobulins IgM, IgG, or IgA. Type III CGs are mixed, the cryoprecipitate containing both polyclonal IgG and IgM. HCV infection is strongly associated with the latter 2 types of MC.
As the clinical manifestations of MC are the consequence of a systemic vasculitis involving mainly the skin, joints, peripheral nervous system, and kidneys, the disease expression (MCS) of CGs is heterogeneous, ranging from the classic triad of mild palpable purpura, weakness, and arthralgia to such life-threatening organ damage as type I membranoproliferative glomerulonephritis (MPGN) and widespread vasculitis with pulmonary hemorrhage, gastrointestinal ischemia, as well as cardiac and CNS involvement. Skin is commonly involved in up to 95% of cases of MCS in the form of cutaneous vasculitis ranging from a palpable purpura and petechiae in the lower extremities to chronic cutaneous ulcers, Raynaud phenomenon, and acrocyanosis. Biopsy of the skin lesions reveals small vessels that are affected by immune complex vasculitis, whereas mononuclear cells heavily infiltrate the vessel walls. Arthralgia without arthritis is common, typically affecting the proximal interphalangeal joints of the hands, metacarpophalangeal joints, knees, and hips. The most frequently reported neurologic manifestation of MCS is a peripheral neuropathy with distal sensory or mixed, sensorimotor polyneuropathy, usually presenting with painful asymmetric paresthesia, numbness, burning, needles-and-pins sensation, skin crawling, and itching that mostly involve hands and feet. At biopsy, axonal damage with epineuronal vasculitic infiltration and endoneuronal microangiopathy has often been demonstrated. Less frequently, multiple mononeuropathies and, even more anecdotally, CNS involvement have been reported. In 30% to 60% of patients with MCS, the kidneys are involved, mostly as an MPGN with subendothelial deposits of proteins. The most frequent clinical presentation of renal involvement during MCS is proteinuria with microscopic hematuria and a variable degree of renal insufficiency. The most characteristic histologic findings are capillary thrombi consisting of precipitated CGs that can be visualized with light microscopy.
The universally accepted pathogenesis of HCV-related MC is a chronic antigenic stimulation of the immune system, which facilitates clonal B-lymphocyte expansion. A possible culprit is an increase of B-cell survival because of the inhibition of apoptosis by Bcl2 activation or by the interaction of HCV E2 envelope protein with the glycoprotein CD81 on the surface of B cells, which ultimately downregulates the activation threshold leading to persistent cell stimulation. The recognition of a molecular mimicry between HCV-specific proteins and certain autoantigens and of NS5A and core proteins of HCV being able to stimulate autoantigen production by the immune system may also account for both B-lymphocyte activation and autoimmune reactions in MCS. All these abnormalities could be the consequence of an altered expression of regulatory microRNAs (miRNAs), as recently suggested by the finding of an overexpression of miRNA 17-92 in the circulating lymphocytes of patients with MC that was reverted by interferon therapy.
Treatment of mixed cryoglobulinemia syndrome
Antiviral therapy with interferon is the mainstay for the long-term control of MCS because HCV RNA suppression leads to interruption of lymphocyte stimulation by HCV and results in an improvement or disappearance of most clinical and laboratory manifestations of virus-related MCS ( Table 1 ). While cumulatively the achievement of a sustained virological response (SVR) to interferon-based therapy has resulted in the recovery from signs and symptoms of MCS in up to 90% of patients, access to interferon therapy was restricted in most patients with advanced hepatitis owing to an increased risk of life-threatening adverse reactions to interferons or myelosuppression. Regrettably, for interferon-ineligible patients, symptomatic treatment of the clinical manifestations of MC without suppressing HCV leads to a transient control of the clinical syndrome only. Thus, virus eradication should be attempted as a first-line therapeutic option in patients with HCV infection with MCS, although either onset or worsening of vasculitic manifestations such as peripheral neuropathy, nephropathy, and skin ulcers have occasionally been reported after administration of interferon. The expected clinical benefit of an SVR is the resolution of polyarthropathy, vasculitis, dermatologic lesions, glomerulonephritis, and proteinuria, whereas most patients with significant renal impairment from HCV-induced glomerulonephritis and those with a peripheral neuropathy with significant neural injury may not fully recover after viral eradication. In a study of pegylated interferon (Peg-IFN)/ribavirin (Rbv) therapy of 253 patients with HCV-associated MC (121 symptomatic), all patients with SVR experienced either a complete or a partial clinical response, and in most responders, MCS disappeared, contrary to all virological nonresponders who were also clinical nonresponders, despite a transient improvement in some cases. Triple therapy with first-generation protease inhibitors has resulted in high rates of virus clearance, yet with substantial toxicity rates. In an open-label prospective single-center cohort study, the safety and efficacy of 48 weeks’ combination therapy with Peg-IFN/Rbv plus an NS3/4A protease inhibitor boceprevir (n = 13) or telaprevir (n = 17) was evaluated in 30 genotype-1-infected patients with severe and/or refractory MC. At week 72, 20 patients (67%) achieved an SVR combined with a complete clinical response, whereas 14 (47%) experienced serious adverse events, all patients being mostly those with advanced liver fibrosis and a low platelet count. In another prospective study, 35 genotype-1-infected patients with MC who received a 48 weeks’ course of Peg-IFN/Rbv plus boceprevir experienced a significant reduction of cryocrit values and an improvement in symptoms; however, they achieved lower SVR rates compared with matched MC-free controls (24% vs 70%; P = .01). Although neither efficacy nor safety data are available for the treatment of HCV-related MC with all oral regimens based on new direct-acting antiviral agents (DAA), high SVR rates and excellent safety and tolerability records can be anticipated with those regimens. However, the demonstration that HCV suppression following direct antiviral therapy restores NK cell function related innate immunity should alert against the clinical risks of immune reconstitution that might also ensue in patients with MCS following exposure to all oral DAA.
| Author, Reference, yr | Treated Patients | Cirrhosis (%) | Genotype 1 (%) | Antiviral Regimen | Duration of Treatment (mo) | Sustained Virological Response (%) | Complete Clinical Response (%) | Adverse Events (%) |
|---|---|---|---|---|---|---|---|---|
| Saadoun et al, 2010 | 55 | Na | 63 | Peg-IFNa2b or 2a/Rbv | 12 | 60 | 73 | 54 |
| Mazzaro et al, 2011 | 86 | 5 | 49 | Peg-IFNa2b/Rbv | 6/12 | 50 | 88 | 9 |
| El Khayat et al, 2012 | 46 | Na | 0 | Peg-IFNa2a/Rbv | 12 | 48 | 48 | 22 |
| Gragnani et al, 2014 | 5 | 100 | 100 | Peg-IFNa2b/Rbv/BOC | 12 | 0 | 0 | 55 |
| Saadoun et al, 2015 | 30 | 40 | 100 | Peg-IFNa2b or 2a/Rbv/PI | 12 | 67 | 67 | 47 |
| Gragnani et al, 2015 | 121 | 29 | 45 | Peg-IFNa2b or 2a/Rbv | 12 | 52 | 50 | 29 |
Targeting the effector arm of MCS with rituximab (RTX), a monoclonal chimeric antibody against B-cell-specific surface antigen CD20, which inhibits B-cell function, has been reported to induce clinical benefits such as a reduction of both CG levels and clinical manifestations. Nevertheless, suppression of B cells by RTX treatment may favor HCV RNA replication, even though this apparently does not significantly harm the liver. After a weekly dosing of 375 mg/m 2 of RTX for 4 consecutive weeks, the vast majority of patients with an MCS could achieve a complete response in the skin, joint, and neuromuscular domain, whereas most patients were able to discontinue corticosteroids. In a randomized controlled trial of 57 patients with MC vasculitis not responding to or unfit for antiviral treatment, RTX showed a better efficacy than such conventional treatments as glucocorticoids, azathioprine, cyclophosphamide, or plasmapheresis. In another study, the administration of RTX was also shown to be safe and associated with clinical improvement in patients with advanced liver disease. When the association of RTX with Peg-IFN/Rbv was evaluated against Peg-IFN/Rbv alone, the former regimen led to more clinical remissions and higher rates of renal response and CG clearance than the RTX-free regimen, but both regimens had comparable rates of safety and clinical and virological adverse events.
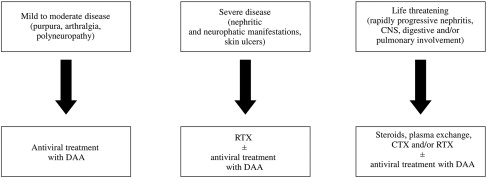
Other options to treat MCS included short-term steroid administration that may help control minor intermittent inflammatory events, yet it is unsuccessful in patients with major disorders such as neuropathy or nephropathy, while it may boost viral replication. Measures such as plasma exchange or administration of antigen-free diets (low-antigen-content [LAC] diet) aimed to stimulate immunocomplex clearance proved to be safe, whereas the administration of such immunosuppressive regimens as cyclophosphamide or azathioprine carry the potential risk of toxicity related to virus reactivation. While an LAC diet has led to improvement of minor manifestations of MC in some patients and therefore is considered the standard of care for the early stages of the syndrome, immunosuppressive regimens are indicated to treat patients with acute, life-threatening manifestations of organ involvement who do not respond to steroids, although these drugs may occasionally cause severe adverse reactions and liver disease progression. Although patients with mild to moderate HCV-related MCS should be prioritized to receive antiviral treatment, RTX is the standard of care for patients with worsening of renal function, neuropathy, skin disease, and intestinal ischemia and needs be initiated before or in parallel with antiviral therapy. Patients with life-threatening organ involvement and those with refractory HCV-related MCS or underlying B-cell NHL need to be prioritized to life-saving options based on steroids, plasma exchange, cyclophosphamide, and/or RTX. In this latter group of patients, antiviral therapy should therefore be deferred ( Fig. 1 ).
Lymphoproliferative disorders
The well-documented, strong association between HCV and B-cell NHL follows a geographic gradient whereby the incidence rates are higher in northern countries than in the southern ones. A most convincing pathogenetic link between HCV and lymphoma was the finding of lower cumulative incidence rates of lymphoma in patients in whom HCV was successfully eradicated following interferon therapy based on a large population study in Japan. In this study, a lymphoma developed in 2.6% of patients with HCV infection over 15 years of observation compared with none among patients who achieved an SVR following interferon-based therapy. The causal role of HCV in the lymphoproliferative disorders is further highlighted by the report of episodes of viral relapse occurring in parallel with lymphoma recurrence. Despite scanty data on HCV replication within B lymphocytes, chronic antigenic stimulation by the virus is thought to trigger B-cell proliferation, resulting in a wide spectrum of injuries ranging from minor expansion of B-cell populations to an aggressive high-grade lymphoma. The most common types of B-cell NHL associated with HCV are lymphoplasmacytic lymphoma; marginal zone lymphoma (MZL), in particular splenic marginal zone lymphomas (SMZLs); and diffuse large B-cell NHL. Approximately two-thirds of the HCV-related NHLs are low-grade tumors with predominantly extranodal involvement of organs such as liver, spleen, salivary glands, and stomach compared with 19% of non-HCV-related lymphomas. Other hematologic disorders in the course of HCV infection include gammopathies of uncertain significance mainly composed of IgM kappa, present in up to 11% of CG-free patients with HCV infection. Clinical surveillance of patients with monoclonal gammopathies is deemed necessary owing to the fact that these patients carry a risk of progressing to multiple myeloma. It should be recognized that MC may be a bridge disorder between HCV infection and several hematological malignancies, a clinical switch that can take place in up to 10% of patients with circulating CGs. B-cell neoplasia more often affects patients with long-standing infections and diagnosis of MC, keeping in mind that HCV infection has been reported in up to 35% of patients with B-cell NHL and in almost 90% of patients with NHL with circulating CGs. The pathogenic mechanism of lymphoproliferative disorders is likely a long-standing infection with HCV resulting in clonal B-cell expansion of CG-secreting lymphocytes and ultimately in a combination of genetic and environmental factors causing a mutational event including activation of oncogenes leading to neoplastic transformation of B cells. Another possible mechanism underlying B-cell transformation during hepatitis C is the inhibition of apoptosis of HCV-infected lymphocytes by (18-14) translocation, which results in an overexpression of the bcl2 oncogene, coupled with a second mutation (myc oncogene) that leads to the development of a lymphoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






