HCV in the East is a complex scenario with prevalence rates of 0.5% to as high as 4.7%, and variable distributions of genotypes, with a dominance of genotype 1b in East Asia, genotype 3 in South Asia and South East Asia, and genotype 6 in Indochina. Approvals for the new oral directing antiviral agents (DAAs), in the East have been very slow, but ultimately will be achieved by 2019, consequently, pegylated interferon and ribavirin are still widely used. Nonetheless the main issues are the problems of screening and linkage to management, and the considerable barriers to access HCV care.
Key points
- •
More than 50% of Hepatitis C carriers worldwide reside in Asia but prevalence is heterogenous with pockets of high and low prevalence, and variable genotype – in East Asia, genotype 1b predominates, while in South and South East Asia, genotype 3 is a dominant genotype, while in Indochina genotype 6 is dominant.
- •
Asians have a much higher prevalence of favourable IL28CC interferon good responder genotype which leads to a high sustained virological response (SVR) rate of over 70%.
- •
The registration approvals of new DAAs lag behind that of Western countries, and with some Asian countries requiring a registration trial, leading to delays of 3–4 years, however, generic licensing and access programs in low income countries will make these drugs more accessible.
- •
Due to the diversity in the epidemiology and genotype distribution, healthcare systems, economic status, reimbursement policies, and access to the new DAAs, a one size fits all strategy is unlikely with the current treatment strategies.
- •
A roadmap for HCV management based on available therapies and their SVR rates provides guidance for treatment selection.
Introduction
More than 170 million persons globally may be infected with HCV, with over 60% living in Asia and an estimated burden of 124 million. In the recent Global Burden of Disease Survey, viral hepatitis accounted for more than 1 million deaths in Asia, of which about 20% are due to chronic hepatitis C. This large burden of disease is compounded by several issues such as inadequate data on disease prevalence, poor screening programs, lack of infrastructure, insufficient number of trained health care personnel, policy inaction, delayed access, and lower priority with regards to health care budgets. Unlike the United States, which is a country, and the European Union, which is a politico-economic union of nations, Asia is made up of a wide variety of nations with a variety of health care systems and gross domestic product (GDP) from the very lowest (Bangladesh GDP per capita $2853) to the highest (Macau $138,025). Hepatitis C disease, like many infectious diseases, is closely linked to the socioeconomic status of the country. In many Asian countries, including some of the most populous ones, which were lower in the list of countries ranked by GDP until recently, not only are health care budgets under duress but priority for viral hepatitis is also low. Consequently, the HCV problem in Asia is a complex one, and each country has specific issues that need to be addressed.
Introduction
More than 170 million persons globally may be infected with HCV, with over 60% living in Asia and an estimated burden of 124 million. In the recent Global Burden of Disease Survey, viral hepatitis accounted for more than 1 million deaths in Asia, of which about 20% are due to chronic hepatitis C. This large burden of disease is compounded by several issues such as inadequate data on disease prevalence, poor screening programs, lack of infrastructure, insufficient number of trained health care personnel, policy inaction, delayed access, and lower priority with regards to health care budgets. Unlike the United States, which is a country, and the European Union, which is a politico-economic union of nations, Asia is made up of a wide variety of nations with a variety of health care systems and gross domestic product (GDP) from the very lowest (Bangladesh GDP per capita $2853) to the highest (Macau $138,025). Hepatitis C disease, like many infectious diseases, is closely linked to the socioeconomic status of the country. In many Asian countries, including some of the most populous ones, which were lower in the list of countries ranked by GDP until recently, not only are health care budgets under duress but priority for viral hepatitis is also low. Consequently, the HCV problem in Asia is a complex one, and each country has specific issues that need to be addressed.
The East as clusters of similar countries
Given the vastness and the variability of the multitude of countries in the East, the Global Burden of Disease Study has grouped countries based on epidemiologic homogeneity so as to allow meaningful health care conclusions to be drawn for countries that are clustered in the same group ( Table 1 ). However, one can also view countries based on their health care reimbursement patterns: full or almost full reimbursement, partial reimbursement, or no reimbursement. Health care reimbursement leads to a single payer and hence a stronger negotiating position with regards to drug costs from pharmaceutical companies. Where there is no single payer, the cost of drugs is set by the pharmaceutical company based on market forces.
| Countries | Health Care Affordability (GDP) | Prevalence (%) | |
|---|---|---|---|
| Group 1: High-income Asia Pacific | Singapore, Japan, South Korea | +++ | 0.5–1.4 |
| Group 2: Central Asia | Armenia, Uzbekistan, Mongolia, Kazakhstan | + to ++ | 3.8 (3.0–4.5) |
| Group 3: East Asia | China, Special Administrative Regions, Taiwan | ++ to +++ | 3.7 (3.4–4.5) |
| Group 4: South Asia | Bangladesh, India, Pakistan | + | 3.4 (2.6–4.4) |
| Group 5: Southeast Asia | Malaysia, Myanmar, Vietnam, Cambodia, Thailand | + to ++ | 2.0 (1.7–2.3) |
| Group 6: Australasia | Australia, New Zealand | +++ | 2.7 (2.2–3.22) |
Varied epidemiology
The true prevalence rate of HCV infection in the East remains less well defined because of the lack of good-quality community studies in many countries. A systematic review of the epidemiology of HCV infection in the East was performed recently. Even within the same country, there may be high discordance in HCV prevalence rates between studies because of the multiethnicity and socioeconomic heterogeneity within the same country. A typical case is Taiwan where a nationwide seroepidemiology survey of 7 townships showed variation of 1.6% in the lowest to 19.6% in the highest. The difference was thought to be related to differences in risk factors, the most common being blood transfusion and unsafe injection practices, acupuncture, and tattooing. In China, studies have reported an estimated prevalence of 1% to 1.9% ; however, a recent seroepidemiology update indicated a much lower prevalence of 0.43%, which was consistent across the different regions in China. One of the reasons for this decrease was the use of newer-generation anti-HCV enzyme-linked immunosorbent assay, which had fewer false-positive results than the previous studies. However, there may be pockets of high prevalence in high-risk groups, such as rural Henan, where paid blood donation is commonly practiced and prevalence rates of up to 9.6% have been reported. In most countries, epidemiologic data are from older serology studies or from blood donor groups, which underestimate the actual prevalence.
Attempts have been made to mathematically predict the actual prevalence in various countries. Using these predictions, the countries with the highest prevalence are those in Central, South, and East Asia (Mongolia, China, Taiwan, and Pakistan), with prevalence rates exceeding 3%. The 2 countries with the highest recorded prevalence are Pakistan (4.7%) and Taiwan (4.4%). Australia, New Zealand, and the Southeast Asian countries are moderate in prevalence (2%–3%). The high-income Asian countries such as Japan, South Korea, and Singapore, having the highest community hygiene standards, have the lowest HCV serology prevalence. Without good prevalence data, prioritization for action is difficult to mobilize from both policy makers and the public. Estimates of disease burden are also misleading.
Complex genotype distribution
The diversity of HCV genotypes in the East is related to the highly heterogeneous ethnicity and routes of transmission in Asia Pacific countries. Genotype 1b is the predominant genotype (45%–64%) in countries in East Asia such as China, Taiwan, South Korea, and Japan, followed by genotype 2. Australia has a fairly equal mix of genotypes 1 and 3 (54% and 37%, respectively). In South Asia and southeastern parts of Asia, the predominant genotype was genotype 3 (45%–79%), in Thailand, India, and Pakistan. Vietnam has a high predominance of genotypes 1 and 6 (30% and 54%, respectively), a pattern that is not shared by other Asian countries. The differences in genotype distribution have significant implications for Asian countries. In particular, genotype 3, the predominant genotype in South Asia and Southeast Asia, now recognized to be associated with worse prognosis, is also a difficult-to-treat genotype. There are more limited therapeutic options for genotype 3 because most of the new all-oral DAAs are active against genotype 1 and they have more limited activity against genotype 3. For Indochina, where genotype 6 is the dominant genotype, there are few studies on antiviral efficacy. These scenarios make recommendations and guidelines of limited value in these countries.
Differing hepatitis C virus treatments
In many Asian countries, the standard of care (SOC) still remains a combination of pegylated interferon and ribavirin (PR), which achieves an SVR in approximately 74% of patients in the largest Asian randomized control study. This situation is attributed to the high prevalence of the IL28B good-responder (CC) genotype in 80% of the patients, the most common genotype in Asians. In contrast, only 50% of Caucasian HCV genotype 1–infected subjects achieve SVR with PR. Unfortunately, most Asians present with advanced disease and many have also failed PR therapy; consequently, there is a large unmet need for new treatments. With the approval of boceprevir, the efficacy of boceprevir triple therapy was evaluated in an early access program of the most difficult-to-treat patient category in Asians, those who had failed therapy and had advanced fibrosis or cirrhosis. The overall sustained virological response at week 12 post treatment (SVR12) was 61% and was similar in both Asian and Caucasian patients, and although there was no mortality in this study, there were significant serious adverse events and adverse events that led to many treatment discontinuations. Consequently, more effective anti-HCV treatments are needed in patients who may otherwise progress to liver cirrhosis and hepatocellular carcinoma (HCC). Although the first-wave DAAs, telaprevir and boceprevir, were approved in the United States and the European Union in 2011, telaprevir was approved only in Japan in Asia. Boceprevir was subsequently approved in many Asian countries, but its use has declined considerably recently. Production of telaprevir has been discontinued, and boceprevir is likely to be discontinued in the near future.
The second-wave DAAs still used an interferon backbone. Simeprevir, peginterferon, and ribavirin as well as sofosbuvir, peginterferon, and ribavirin were approved for treatment of genotype 1 in the United States and European Union. The phase 3 studies were performed in the United States and European Union and had few Asian patients, with the exception of Japan. Although the efficacy of these drugs was superior to that of the first-wave DAAs, the responses were still less than ideal and came with adverse events because of the use of peginterferon and ribavirin. In Asia, simeprevir has been approved only in Japan at a reduced dose of 100 mg/d with PR for 24 weeks. This regimen led to SVR rates of 88.6% in treatment-naive genotype 1–infected patients, similar to Western studies in which simeprevir 150 mg/d with PR achieves SVR rates of 80%. Studies on sofosbuvir and PR for 12 weeks achieved high rates of SVR in Western studies in treatment-naive patients, but there are no randomized control trials on treatment-experienced patients, particularly in the more difficult-to-treat group of treatment-experienced patients with cirrhosis. Real-world data from HCV TRIO suggest that this is a suboptimal regimen in such patients. In Asia, approval has lagged behind Western countries; although registration has been filed in multiple counties, approval has been limited to a few Asian countries ( Table 2 ), but is expected to be complete in Asia by 2018–2019. A roadmap to guide clinicians on the efficacy of various available therapies can be viewed in Fig. 1 .
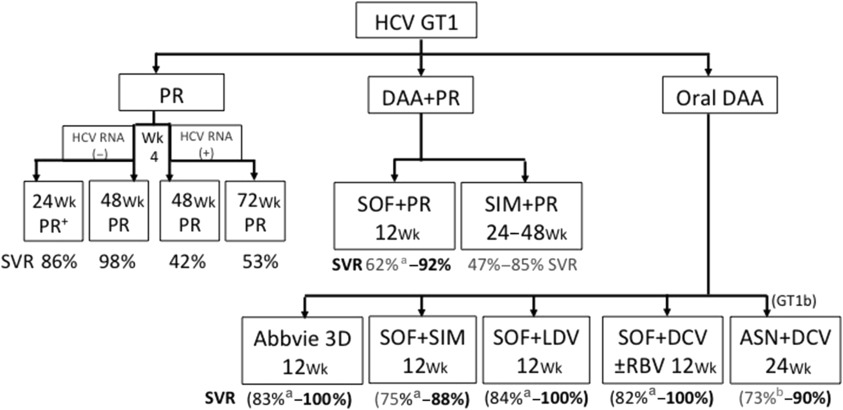
There have been limited studies with the all-oral DAAs in Asia. One of the few DAA combinations to be tested in Asia was the all-oral combination of asunaprevir and daclatasvir, which has been approved in Japan but can only be used for genotype 1b, a strain that is by far the most common genotype 1 subtype in Asia. In a phase 3 international study (HALLMARK-DUAL), 307 treatment-naïve nonresponders to PR or PR intolerant genotype 1b–infected patients were treated with asuneprevir and daclatasvir for 12 or 24 weeks. SVR12 was 90% in patients in the treatment-naive cohort, 82% in the nonresponder cohort, and 82% in the ineligible, intolerant, or ineligible and intolerant cohort, 168 (82%; 95% CI, 77–87) in the nonresponder cohort, and 192 (82%; 95% CI, 77–87) in the ineligible, intolerant, or ineligible and intolerant cohort. An open-label study of genotype 1b–infected patients (interferon-ineligible/intolerant patients and nonresponders) was performed in Japan. Sustained virological response at week 24 post treatment was achieved by 87.4% of interferon-ineligible/intolerant patients and 80.5% of nonresponder (null and partial) patients; rates were similar in patients with cirrhosis (90.9%) and without cirrhosis (84.0%). The combination of asuneprevir and daclatasvir was approved for treatment of genotype 1b chronic hepatitis C in Japan in July 2014, being the first DAA all-oral combination to achieve initial global registration in Asia ; this was largely because of the high efficacy of the combination in genotype 1b infection, which is by far the predominant genotype 1 subtype in Japan. The combination has substantially lower efficacy in genotype 1a infection and is prone to develop drug-resistant mutations. Other recently approved DAAs, such as sofosbuvir, sofosbuvir and ledipasvir (Harvoni), and Abbvie 3D combination ± ribavirin (Viekira Pak), have not been tested in the East, and most studies of these DAAs have few Asian patients. However, there is no reason to suspect that the efficacy of these DAAs are likely to be different from that of the Western phase 3 studies. The efficacy of these drugs in different HCV-infected populations has been updated in the most recent European Association for Study of Liver (EASL) clinical practice guidelines.
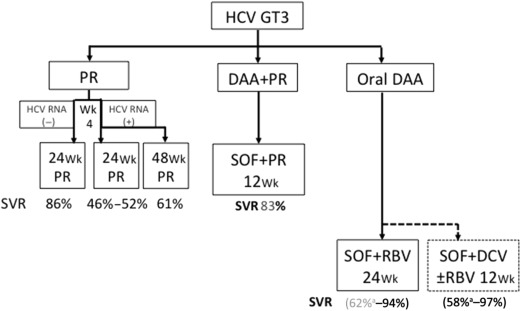
Genotype 3 poses a major problem in South Asia and Southeast Asia, where it is the dominant genotype in some countries such as Pakistan. Treatment with PR for 24 weeks is the SOC in most Asian countries, although treatment extension to 48 weeks is common for those with cirrhosis. Unfortunately, there are not much clinical trial data to support this strategy, and if patients are positive for HCV RNA at week 4, SVR rates seem suboptimal. Sofosbuvir and daclatasvir for 12 weeks is also an option but as daclatasvir is not readily available in Asia, this may not be a suitable option; sofosbuvir and ribavirin for 24 weeks can be used in treatment-experienced patients with cirrhosis ( Fig. 2 ), but this has poor efficacy (60% SVR). However, this strategy may still be useful in Asia for treatment-naive patients even with cirrhosis despite the lack of recommendation from EASL guidelines.