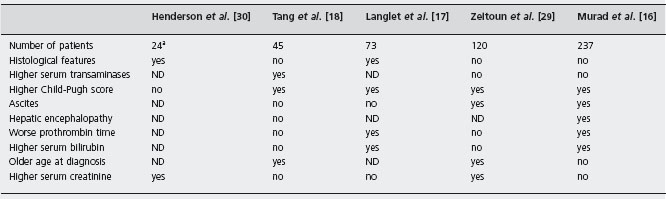
MPD: Myeloproliferative disorders.
PHN: Parosysmal nocturnal hemoglobinuria.
aPatients who had shunt.
ND: not determined.
Treatment
Medical therapy and management of complications
All patients should receive anticoagulation, unless contraindicated, starting with intravenous heparin, then warfarin, to maintain the international normalized ratio of at least 2.5, if not higher. This treatment will control the disease in 10% of cases when the thrombosis is mild and prevent progression, although there are no randomized trials [17,31,34].
Fulminant BCS is usually associated with abundant necrosis that ideally requires liver transplantation. Even in the setting of immediate shunting (surgical or TIPS), hepatic regeneration will rarely take place and liver transplantation must be available as the liver failure may worsen [35]. In patients with acute BCS, early thrombolytic therapy used within 72 hours from diagnosis and infused directly into the thrombosed hepatic vein for 24 hours, has had variable success [36]. Adjunctive angioplasty or stent placement may not be of further therapeutic benefit [37].
Angioplasty
Short-segment obstruction or webs in hepatic veins or the IVC are treated successfully by balloon dilatation or intravascular stents. Membranous vena caval obstruction can be relieved initially in 90% of patients, but 20–30% will need additional angioplasty [38, 39]. Eapen et al. reported 94% and 87% survival at one and five years in BCS patients with mild disease, according to the Murad classification, who were treated only by radiological intervention at a single center [40].
Approximately half of all cases of rethrombosis are due to sub optimal antic oagulation therapy. When there is diffuse thrombosis of hepatic veins, angioplasty alone is only successful in 56% of patients, even with additional thrombolytic therapy. On the other hand, stents result in long-term patency rates of 80–90%, requiring further angioplasty in 50% [41]. Failure of thrombolysis or angioplasty and the presence of a diffuse hepatic vein thrombosis are indications for shunting.
Portosystemic shunting
The therapeutic principle of portosystemic shunting is the conversion of the portal vein into an outflow tract (reversed portal flow), thus decompressing the sinusoids. Thrombosis or compression of the IVC maintains a high pressure in the infrahepatic IVC. Therefore a portacaval or mesocaval shunt will not provide decompression, whereas a shunt from the portal or mesenteric vein to the suprahepatic IVC or right atrium will be effective.
Surgical shunts Patients with a non-fulminant presentation of BCS and those without significant hepatic fibrosis who have a chronic presentation, can be considered for surgical shunting, providing the portal vein is patent. A side-to-side portal caval shunt (or meso-caval shunt) not only decompresses the liver, but also relieves ascites and eliminates the risk of variceal bleeding. A differential of 10mmHg or more between the portal and intrahepatic IVC is considered essential [32]. A hypertrophied caudate lobe often makes the side-to-side portal caval shunt difficult to construct. Orloff et al. reported no technical failures, one operative death, 95% survival, a complete resolution of ascites and no encephalopathy in 60 patients, followed for a range of 3.5 to 27 years. Normal liver histology was found in 48% of patients, and stable fibrosis in the other patients, but cirrhosis did not regress. All shunts, with the exception of two patients who experienced late thrombosis, remained hemodynamically effective, despite some patients not receiving anticoagulation [42]. Contrary to this study, another series reported a survival rate between 57–75% [35, 43] suggesting Orloff et al. either possessed greater experience or used more favorable selection criteria.
If the IVC is patent but severely compressed, a self-expanding stent can be placed in the intrahepatic IVC, followed by a surgical infrahepatic shunt [44]. Retrospective series have questioned the benefits of surgical shunts due to the lack of a survival advantage, independent of liver disease severity, the type of shunt (including TIPS), and the interval between diagnosis and procedure; an exception was patients with mild hepatic impairment (82% vs 68% survival at five years) [19,21]. This lack of effect on survival could be explained by the average 25% hospital/perioperative mortality (range 0–30%) [35], and late shunt dysfunction/thrombosis [45]. Increased fibrosis at surgery was associated with higher mortality [32]. Some perioperative mortality can be attributed to acute hepatic decompensation, which is remedied only by emergency salvage liver transplantation.
Thus, shunting should be performed in a liver transplantation center where rescue therapy is possible. Moreover a TIPS procedure should be attempted before a surgical one, allowing a trial of shunting; if this fails, a liver transplantation is indicated. B4
Transjugular intrahepatic portosystemic shunts (TIPS) The use of TIPS has improved the management of BCS. TIPS avoids laparotomy, overcomes caudate lobe compression and occlusion of the IVC, with less periprocedure mortality than surgical shunting, particularly in patients with poor liver function. Using TIPS does not preclude subsequent surgical shunting or liver transplantation [14, 46–48]. With TIPS, the porto-caval pressure gradient should be normalized (≤6mmHg). In three series, mortality rates ranged from 9–30% during a mean follow-up of four years. Among 65 patients, seven died: one with fulminant liver failure (transplantation contraindicated), four with severe liver disease (acute on chronic presentation) and three with underlying hematological disorders.
Long-term patency, despite routine anticoagulation therapy, only averaged 50%, with 36–72% of patients needing reintervention. TIPS can be placed even if there is portal vein thrombosis [11]. Polytetrafluoroethylene-covered stents result in patency rates of 67% at one year compared with 19% with uncovered stents, a very low rate compared with other series [46]. A recent cohort study on 124 patients with BCS who underwent TIPS in six centers in Europe showed that TIPS was able to improve survival according to Rotterdam score prediction with five years survival of 71% in high risk patients [49].
TIPS should be considered as first-line therapy, if variceal bleeding occurs, for acute and chronic BCS and also in patients with fulminant BCS if a liver donor is not available within 2–3 days. B4 A recent report demonstrated that among five patients with FHF, TIPS allowed resolution of the disease in one, and acted as a bridge to liver transplantation within one month in three patients [50]. TIPS intervention should be managed carefully if the patient’s liver reserve is sub-optimal and rescue liver transplantation should be available. B4
Legend: ND: not determined.
Liver transplantation
In the remaining 10% of patients, when percutaneous angioplasty or TIPS fail, liver transplantation should be considered. Emergency liver transplantation is indicated for fulminant BCS, and has been used as a salvage procedure for fulminant liver failure induced by surgical shunting [35, 43,51].
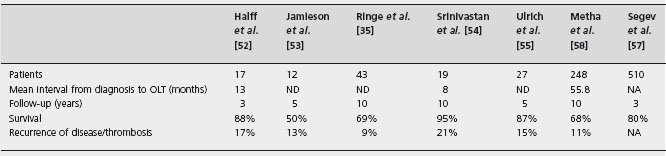
There are seven major transplant series, with more than ten patients each, totaling 162 patients (see Table 37.3), with survival rates between 50% and 95% with a mean follow-up of 4.5 years [35,52–55]. Twenty patients (12%) had acute portal vein or hepatic arterial thrombosis, or late thrombosis, which was fatal in eight and resulted in retransplantation in eight. Despite anticoagulation recurrent BCS occurred in two of seven transplanted patients [56].
Recently a retrospective analysis of series from USA and Europe has shown a five-year survival rate of 80% [57, 58]. However, a TIPS was placed in only 4% of patients before transplant in the European series and anticoagulation was used in less than 60%. Recurrence of venous thrombosis or BCS occurred in about 10% of patients, confirming the need of anticoagulation.
Although almost all genetic thrombophilic disorders are cured by transplantation [43, 59], thrombosis still occurs and routine anticoagulation therapy is necessary. Careful monitoring is necessary as complications of anticoagulation were seen in 40% and in 17/197 (11%) after liver transplantation who had bleeding complications due to anticoagulation leading to two deaths in the European series [56].
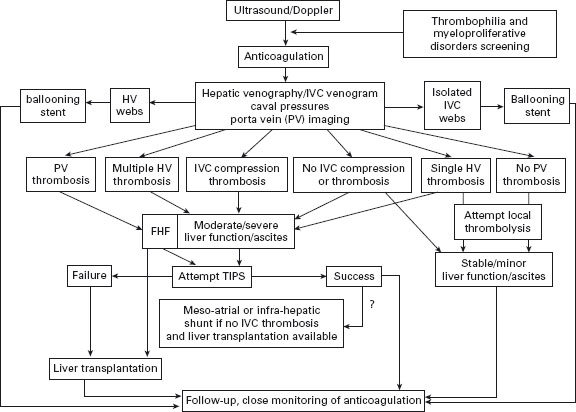
In patients with myeloproliferative disorders, use of hydroxyurea and aspirin is safe and effective [60]. So far malignant transformation is anecdotal [54,58]. A suggested algorithm of treatment for BCS is shown in Figure 37.1.
Portal and splanchnic vein thrombosis
Splanchnic thrombotic disease has a wide spectrum of presentation and a wide range of severity. The disorders can be challenging and sometimes overlapping; appropriate management hinges on accurate diagnosis and especially on the broad distinction between cirrhosis-associated thrombotic disease and non-cirrhotic splanchnic venous thrombosis. The complexity of these cases, especially with non-cirrhotic acute PVT or cavernoma, usually warrants a multidisciplinary approach with involvement of both hematology and GI/hepatology, as well as other support services. Below, we have focused on portal vein thrombosis (PVT) and isolated splenic vein thrombosis (SVT), with emphasis on adult disease from a practical clinical perspective. Several recent reviews also offer excellent and comprehensive examination of the disorder [61–63].
Portal vein thrombosis (PVT): epidemiology
The exact annual incidence of PVT is not known although it is a common condition in most tertiary care centers. The difficulty in determining clear epidemiology of PVT results in part from its highly variable associations. In an autopsy series, PVT was discovered in 254 of 23,796 autopsies (1%) performed over a 12-year period from Uppsala Sweden [64]. Among those with PVT, 28% had cirrhosis (one-third with and two-thirds without hepatocellular cancer), 23% had primary hepatobiliary cancer (two-thirds hepatocellular cancer and one-third biliary), 44% had metastatic cancer, 10% had intra-abdominal infection or inflammatory conditions, 3% had myeloproliferative disease and 14% were without an apparent association. As discussed below, up to 20% of patients with cirrhosis develop portal thrombosis, which probably constitutes the single most common association encountered in liver centers. However, PVT in cirrhosis is frequently discovered incidentally and, while it may result in liver atrophy and warrants a close examination for an associated hepatocellular cancer, it is a less urgent clinical situation than acute PVT in the non-cirrhotic patient in whom the presentation can be dramatic.
In all cases, additional diagnostic investigation is warranted and the risk-benefit of anticoagulation therapy needs to be carefully considered. However, as a result of the broad array of presentations and relatively low prevalence, controlled data treatment is limited and much of the literature, including two efforts at consensus or summary statements, are reflections more of experience and expert opinion than evidence-based on randomized trials [65,66].
Portal vein thrombosis: overview of clinical presentation and evaluation
The typical clinical presentation ranges from sudden onset of abdominal pain and ascites with acute PVT, to acute variceal hemorrhage in the setting of chronic PVT, to incidentally discovered cavernoma (cavernous transformation of the portal vein) found, for example, during an evaluation of low platelets due to hypersplenism and splenomegaly. These clinical situations can present the clinician with diagnostic and therapeutic problems which need to be systematically worked through. For example, the presence of splenomegaly may be both part of PVT secondary to the thrombus and localized portal hypertension and also part of an underlying myeloproliferative disorder. Ascites can be seen even in patients without cirrhosis and its evaluation can be confusing (see below).
These studies, especially CT and MR, may also help to establish whether the thrombus is recent (absence of collateralisation) or chronic (presence of cavernous transformation.) Long standing portal vein thrombosis may result in irregularity of the liver outline, probably due to asymmetry of blood flow, resulting in the radiological appearance of cirrhosis-in these instances a biopsy is required, as the prognosis will be significantly altered by the histology.
The clinician then needs to exclude the presence of varices by endoscopy which then helps to weigh the risk-benefit of anticoagulation therapy. Laboratory tests should be performed to evaluate for hypercoagulable conditions and the history must be carefully explored to evaluate for any evidence of prior liver abnormalities, use of prothrombotic agents such as estrogen preparations, and prior thrombotic disease, including family history and prior intra-abdominal inflammation. Detailed questioning is needed regarding any history of neonatal illness which would suggest possible umbilical vein cannulation, and any distant episodes of intra-abdominal illnesses which might suggest prior appendicitis or diverticulitis.
Acute non-cirrhotic PVT
Acute, non-cirrhotic PVT is the least common cause of PVT but perhaps the most dramatic. Among 3655 liver disease patients recorded in a University of Virginia liver disease registry kept between approximately 1995 and 2004, 19 patients (0.5%) presented with this condition (unpublished observations). Although these patients have not yet been thoroughly evaluated about one-third of these had an underlying myeloproliferative disorder and another third had an identifiable pro-thrombotic condition. The most common presentation in our patients was abdominal pain and ascites, with intractable variceal bleeding in a minority. Similarly, Condat et al. observed significantly less gastrointestinal bleeding in acute PVT compared to cavernoma (see below) [67]. Bloody diarrhea may also be evident, especially if the thrombosis extends into the superior mesenteric vein distribution. Ascites can also be seen in one-third of patients with non-cirrhotic acute PVT [61]. Opening of portosystemic collaterals dissipates the portal hypertension and thus the ascites, although a transition to chylous ascites indicates rupture of the pressurized lymphatic vessels [68]. The ascites fluid characteristics may be difficult to interpret as the fluid (in non-chylous situations) derives from bowel edema rather than the typical liver-derived transudate as in cirrhosis [69].
Although 20–40% of patients have no identifiable underlying thrombophilic risk factors and there is a degree of regional variation, myeloproliferative disorders (polycythemia vera, essential thrombocythemia or myelofibrosis) are the most common identifiable causes of acute PVT, while specific factor abnormalities such as protein C, S or anti-thrombin III deficiency, factor V Leiden or prothrombin mutation, and anti-phospholipid abnormalities are associated risk-factor in the remaining cases [67, 70].
Myeloproliferative disorders underlie PVT more often than previously thought, and it is here that the test for the JAK2 mutation has been particularly eye-opening, as it has provided a non-invasive screening test for bone marrow disorders allowing one to select patients for the more invasive bone marrow biopsy [71]. This test probably should become a part of the routine laboratory evaluation of these patients but this remains to be determined. It should be considered if available.
Morbidity in acute PVT can be substantial, but prolonged survival in acute non-cirrhotic PVT is common. Consistent with our own experience, Janssen et al. reported ten-year survival of 81% in this group provided that cirrhosis and underlying malignancy were excluded [72]. As discussed further below, long-term morbidity related to recurrent thrombi and/or transformation to cavernoma can be influenced by early anticoagulation, although controlled data is lacking, and the benefits must be weighed against the risk of severe portal hypertensive bleeding and other complications of anticoagulation.
Portal cavernoma
This entity was first reporteded in 1869 by Balfour and Stewart to describe the post-thrombotic dilatation of the portal vein, which we now know to be commonly associated with prior and often unrecognized PVT. The term “cavernoma” was subsequently used by Köbrich in 1928 to describe the spongy appearance of the network of blood vessels in the portal vein location related to re-canalization of the thrombosis. Local infections (portal phlebitis, omphalitis), abdominal trauma and a history of prothrombotic disorders are implicated in the development of the PVT, but many cases remain unexplained. Cavernoma develops as the portal vein thrombus undergoes reorganization and remodeling into a network of collateral vessels which overcome the occlusion and restore, to a variable degree, hepatopedal blood flow. Cavernous transformation can occur rapidly even within 6–20 days and thus the presence of cavernoma, while usually suggesting chronicity, does not necessarily indicate a very distant event [73]. Hematemasis and splenomegaly are the most common presenting symptoms occurring in about 50% of these patients [74].
Portal biliopathy (biliary obstruction and stricturing) due to ischemia, duct compression by collateral vessels or possible local infection causes symptomatic disease in 4 to 24%, although abnormal cholestatic liver chemistries and cholangiographic abnormalities are much more common [75]. Treatment options include medical therapy with ursodeoxycholic acid, endoscopic therapy including biliary stenting, and in severe cases surgical or radiological shunting. In chronic cases, pre-operative decompression of the portal cavernoma is being explored to avoid life-threatening hemorrhage and other complications following surgical intervention [76]; decompression can be feasible by the placement of TIPS [77].
Strategies to avoid the complications of portal cavernomas have involved early anticoagulation, in cases with early diagnosis, to promote recanalization [67]. Long-term therapy is essential in those with an identified pre-thrombotic condition such as underlying protein C or S deficiency. However, intervention with anticoagulation requires careful exclusion and management of varices if present. It is also important to recall that portal cavernoma may be stable over many years, even without aggressive intervention, especially among patients presenting with more indolent symptoms, which is also our experience. In such cases, we have typically used low-dose forms of anti-coagulation provided varices have been excluded endoscopically.
PVT in cirrhosis
Portal vein thrombosis (PVT) occurs in 10–20% of all cirrhotic patients, and conversely cirrhosis is responsible for about 20% of all cases of PVT [78]. Symptoms of acute abdominal pain, variceal bleeding, and ascites develop in 57% of cirrhotic patients with PVT [79]. Portal vein thrombosis in this setting can develop due to hepatic fibrosis and the resistance to blood flow (stasis), but there can also be many other factors, including a genetic predisposition to prothrombotic disorders, or abdominal infections, or local factors, for example pancreatitis [80]. Thrombosis can extend distally into right and left portal vein branches or proximally into the superior mesenteric vein (see Table 37.4). More often the thrombosis in the portal vein is incomplete and does not result in complete occlusion [81].
Complications of PVT in cirrhosis include splenomegaly, esophageal varices, gastric varices, and portal hypertensive gastropathy. PVT has traditionally been a contraindication to liver transplantation due to technical difficulties in intra-operative anastomases. This situation has led to evaluation of a trial of anticoagulation in patients with cirrhosis and PVT [82]. However, monitoring anticoagulation in cirrhosis with an underlying coagulopathy is a challenge. Traditional measures such as INR (international normalized ratio) are of limited use because it is not only affected by oral anticoagulation medication, such as coumadin, but also factor deficiencies from hepatic dysfunction.
Low weight molecular heparins may be safer than oral anticoagulation in cirrhosis. Recently, repermeation was obtained in 50% of 38 patients with cirrhosis and portal vein thrombosis with only one episode of non-severe variceal bleeding [83].
Further study is clearly essential to determine the relative utility and the risk-benefit of this type of intervention. Surgical techniques involving thrombectomy and bypass grafts also remain to be fully evaluated but should be used cautiously as these may be associated with high morbidity and mortality [84].
Table 37.4 Grades of portal vein thrombosis (PVT).
| Grade | Description |
| Grade I PVT | Thrombosis of intrahepatic portal vein branches |
| Grade 2 PVT | Thrombosis of right or left portal branches |
| Grade 3 PVT | Partial obstruction of portal vein trunk |
| Grade 4 PVT |