Iron removed by venesection
Historically hemochromatosis was diagnosed when symptoms developed in the fifth or sixth decade and patients had significant iron overload at the time of diagnosis. The removal of 500 ml of blood weekly (0.25 g iron) was well tolerated, often for years, without the development of significant anemia. If a patient became anemic (hemoglobin < 100 g/l) after only six venesections, this suggested mild iron overload incompatible with the diagnosis of hereditary hemochromatosis. We now know that these guidelines no longer apply as population and pedigree studies uncover patients in their second and third decade [16]. At our center only 71% of homozygotes would have met the arbitrary criterion that more than 5g of iron (20 venesections) were removed without anemia [17]. This historical diagnostic criterion for hemochromatosis is no longer relevant in the era of genetic testing. B4
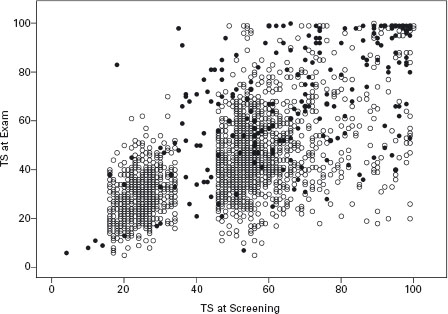
Figure 30.1 A comparison of the transferrin saturation at initial screening (random) and at the clinical examination (fasting) in all participants recalled for a clinical exam (• = C282Y homozygotes, o = non-C282Y homozygote, n = 2285). The apparent gap around 40% is related to the requirement of control participants to have a TS between the 25th and 75th percentile (reprinted with permission from the Am J Med).
Liver biopsy
Liver biopsy has been the “gold standard” diagnostic test for hemochromatosis, but its use has shifted from a diagnostic tool to staging the degree of fibrosis. The need for liver biopsy seems less apparent in the young asymptomatic patient with a low clinical suspicion of cirrhosis based on history, physical examination and iron studies. Clinical guidelines have been suggested, such as a serum ferritin < 1000 micrograms/l or age < 40 years to reduce the need for liver biopsy [18]. Clinical judgment and assessment of concomitant risk factors (alcohol, viral hepatitis) would be a better guide for the need for liver biopsy than an arbitrary threshold. Most non-cirrhotic patients with hemochromatosis have serum ferritin < 1000 micrograms/l and a normal aspartate transaminase (AST) [19, 20]. Cirrhosis can be predicted non-invasively in C282Y homozygotes if the serum ferritin is >1000 micrograms/l, the platelet count is less than 200 × 106/l and the AST is >40U/l [21]. The presence of fibrosis can also be assessed using hepatic elastography [22].
Patients with cirrhosis have a 5.5-fold relative risk of death compared with non-cirrhotic hemochromatosis patients [23, 24]. Cirrhotic patients are also at risk of hepatocellular carcinoma. The mean age of cirrhotic patients with hepatocellular carcinoma was 68 years in a Canadian series, but was lower in Italian patients with concomitant viral hepatitis [25]. Although early detection of hepatocellular cancer has been clearly demonstrated by serial ultrasound and α-fetoprotein determination, curative treatment options remain limited. An elderly cirrhotic patient may not withstand a major resection and the residual cirrhotic liver remains a fertile ground for new tumor development. Organ shortages often preclude the possibility of immediate liver transplantation, although living related adult liver transplantation may improve this situation in the future.
Hepatic iron concentration and hepatic iron index
The traditional method of assessing iron status by liver biopsy uses the semi-quantitative staining method of Perls. However, when moderate iron overload is present, the degree of iron overload can be difficult to interpret. Iron concentration can be measured using atomic absorption spectrophotometry. This can be done on a piece of paraffin embedded tissue, so special preparation is not required at the time of the biopsy. An advantage of cutting the tissue from the block is that one can be more certain that the tissue assayed is the same as the tissue examined microscopically. The normal reference range for hepatic iron concentration is 0–35μmol/g dry weight (<2000 micrograms/g). The hepatic iron index has therefore limited use with the advent of genetic testing. The commentary on liver biopsy reports that a hepatic iron index >1.9 confirms a diagnosis of genetic hemochromatosis should be strongly discouraged.
Imaging studies of the liver
Magnetic resonance imaging (MRI) can demonstrate moderate to severe iron overload of the liver. The technology is advancing and it is possible that eventually it may become as precise as hepatic iron determination [26,27]. Proponents of MRI have emphasized the non-invasive nature of the test for the diagnosis and alleviated need for liver biopsy. As previously discussed, the role of liver biopsy has now shifted from a diagnostic tool to a prognostic tool. It is likely that the presence of an elevated ferritin with a positive genetic test will satisfy the non-invasive clinician more than an MRI study. MRI can also demonstrate the clinical features of cirrhosis such as nodularity of the liver, ascites, portal hypertension and splenomegaly as well as hepatocellular carcinoma. These features can be more readily assessed by abdominal ultrasound at a lower cost.
Genetic testing for hemochromatosis
A major advance, which stems from the discovery of the HFE gene, is the diagnostic genetic test. The original publication reported that 83% of a group of patients with suspected hemochromatosis had the characteristic C282Y mutation of the HFE gene. In this report, the gene was called HLA-H but this name was later changed to HFE [1]. The C282Y mutation is also reported as 845A in some laboratories, reflecting the base pair change rather than the amino acid change. Subsequent studies in well-defined hemochromatosis pedigrees reported that 90–100% of typical hemochromatosis patients had the C282Y mutation [28]. The presence of a single mutation in most patients was in marked contrast to other genetic diseases in which multiple mutations have been discovered (cystic fibrosis, Wilson’s disease, α-1-antitrypsin deficiency). A second minor mutation, H63D, was also described in the original report [1]. This mutation does not cause the same intracellular trafficking defect of the HFE protein and many homozygotes for H63D have been found without iron overload in the general population. Compound heterozygotes (C282Y/H63D) and H63D homozygotes (H63D/ H63D) may resemble homozygotes with mild to moderate iron overload, particularly if a co-factor is present, for example NAFLD, HCV, or alcohol. However, these compound heterozygote (C282Y/H63D) patients usually have normal iron studies [9, 29].
The interpretation of the genetic test in several settings is shown in Box 30.1. The test may also be performed on DNA extracted from paraffin embedded tissue such as liver explants. Studies of explanted livers have demonstrated that many liver transplant patients classified as hemochromatosis patients are negative for the C282Y mutation [30]. This suggests that those patients may have had iron overload secondary to chronic liver disease rather than hemochromatosis. Therefore any interpretation of iron reaccumulation post liver transplant for hemochromatosis must be done with caution and with the benefit of genetic testing.
BOX 30.1 Interpretation of C282Y, genetic testing for hemochromatosis
C282Y homozygote: This is the classic genetic pattern that is seen in >90% of typical cases. Expression of disease ranges from no evidence of iron overload to massive iron overload with organ dysfunction. Siblings have a one in four chance of being affected and should have genetic testing. For children to be affected the other parent must be at least a heterozygote. If iron studies are normal, false positive genetic testing or a non-expressing homozygote should be considered.
C282Y/H63D (compound heterozygote): This patient carries one copy of the major mutation and one copy of the minor mutation. Most patients with this genetic pattern have normal iron studies. A small percentage of compound heterozygotes have been found to have mild to moderate iron overload. Severe iron overload is usually seen in the setting of another concomitant risk factor (alcoholism, viral hepatitis, NAFLD).
C282Y heterozygote: This patient carries one copy of the major mutation. This pattern is seen in about 10% of the Caucasian population and is usually associated with normal or mildly increased iron studies. In rare cases the iron studies are high in the range expected in a homozygote rather than a heterozygote. These cases may carry an unknown hemochromatosis mutation and measurement of body iron stores is helpful to determine the need for venesection therapy.
H63D homozyote: This patient carries two copies of the minor mutation: Most patients with this genetic pattern have normal iron studies. A small percentage of these cases have been found to have mild to moderate iron overload. Severe iron overload is usually seen in the setting of another concomitant risk factor (alcoholism, or viral hepatitis).
H63D heterozygote: This patient carries one copy of the minor mutation. This pattern is seen in about 20% of the Caucasian population and is usually associated with normal iron studies. This pattern is so common in the general population that the presence of iron overload may be related to another risk factor. Liver biopsy may be required to determine the cause of the iron overload and the need for treatment in these cases.
No HFE mutations: There are currently some newly recognized mutations associated with iron overload that are being studied in research laboratories (ferroportin, transferrin receptor 2, hepcidin, hemojuvelin). There will likely be other hemochromatosis mutations discovered in the future. If iron overload is present without any HFE mutations, a careful history for other risk factors must be reviewed and liver biopsy may be useful to determine the cause of the iron overload and the need for treatment. Many of these cases are isolated, non-familial cases.
Non-expressing C282Y homozygotes:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







