div class=”ChapterContextInformation”>
15. Wound Healing and Plastic Surgery Principles
Introduction
Successful surgery relies on proper wound healing and tissue repair. When these processes do not follow their normal pattern, urological surgeons may be faced with complications including skin separation, wound dehiscence, tissue necrosis, and breakdown of vascular, urinary and parenchymal structures. Determinants of proper surgical healing include preservation of critical anatomical structures; restoration of tissue integrity and homeostasis; and the regenerative potential of tissues. In addition, the surgeon must have a detailed knowledge of previous surgical, traumatic, infectious, and radiation related perturbations to normal anatomy and physiology, along with an understanding of the time course and vulnerability of tissues after injury in order to properly plan for successful primary repair or tissue transfer to achieve restoration of function.
gross anatomy with particular emphasis on the vascular supply of skin, subcutaneous tissue, fascia, muscle and individual organs.
understanding of the microvasculature of skin and epithelia, which support the primary barrier functions of the body.
cellular and tissue events in restoring tissue integrity.
proper harvesting of free grafts and the process and timeline of tissue engraftment, for cases when tissue transfer cannot be accomplished with a vascularized flap.
flap creation, which relies on the intrinsic vascular anatomy of the tissue, propensity for collateral formation, and in cases of replantation or free flaps, the timeline for perfusion and reperfusion.
Gross and Vascular Anatomy
Genitourinary tract organs vary in their response to injury and “success” in wound healing in proportion to the density and/or redundancy of their blood supply. In any organ, matched arterial inflow and venous outflow are required for wound healing, and congestion of the venous outflow can just as certainly, although more slowly, doom the success of a tissue transfer or surgical procedure as arterial occlusion. In this section, we will work from cephalad to caudad to review how anatomical knowledge informs surgical principles and patient care.

Ureteral vascular architecture . (a) schematic drawing of vascular plexus (drawn from Ref. [3]) demonstrating adventitial vascular plexus; perforators; and mucosal vascular plexus. 1- urothelium, 2 – muscular wall, 3 – adventitia, 5 – segmental arterial and venous supply, 6 – adventitial vascular plexus, 7 – perforating arteries, 8 – mucosal vascular plexus. (b) ureteral vascular regions and impact on reconstruction
Ureteral surgery depends on the vascular network within the ureteral wall for its safe mobilization and effective use in reconstruction (Fig. 15.1) [3]. An adventitial vascular plexus receives arterial supply from a number of points along the ureteral course including the renal artery, the aorta, iliac artery and its pelvic branches. These perforate through the muscular layers to reach the mucosal vascular plexus . The ureter thus has a variable propensity for ischemia like any flap; ureteral healing depending on the amount of mobilization, the distance from its next perforating arterial branch, and preexisting or intraoperative damage to its vascularity from fibrosis, radiation or improper manipulation. These considerations greatly influence the choice of ureteral reconstructive strategy, from pyeloplasty and ureteroureterostomy when both ends are healthy, to reimplantation or substitution. Figure 15.1 provides the divisions of ureteral vascular zones and appropriate reconstructive strategies. The portion of the ureter crossing the iliac vessels and more distal is considered in a watershed area, and less reliably supports ureteroureterostomy; reimplantation is preferred whenever possible in the lower 1/3 of its course.
The bladder and prostate are resistant to ischemic complications, in part because of redundant blood supply (see chapter on anatomy of the pelvic organs), a relatively low metabolic demand compared to other organs (such as the kidney or testis) and the robust interconnected intrinsic vasculature which allows for collateral flow beneath the epithelium. Bladder ischemic damage results from longstanding outlet obstruction, chronic inflammation or pelvic irradiation. Its usual manifestation is in a loss of compliance and elasticity, making it difficult to reconfigure for reconstructive purposes such as a Psoas hitch, Boari flap, Y–V plasty or bladder tube formation. Circumstances in which prostatic ischemia are relevant to wound healing predominantly relate to prior radiation therapy [4, 5], in which obliterative endarteritis leads to inadequate reepithelialization of the prostatic fossa and development of fibrosis, heterotopic calcifications and refractory stenoses. Rarely, severe ischemic complications of pelvic fracture including vascular injury or embolization may lead to prostatic or membranous urethral necrosis.
The male external genitalia and urethra benefit from a highly redundant vascular supply possibly reflecting the evolutionary importance of reproductive success [6]. The posterior urethra receives inflow through branches of the inferior vesical artery perforating through the bladder neck and prostate, while the anterior urethra is served by multiple branches of the internal pudendal artery. These include bilateral direct flow from the bulbourethral, with important collateral supply from the dorsal arteries (via anastomoses in the glans) as well the deep or cavernosal artery (via perforators from the corpora cavernosa to the spongiosum). Importantly, the watershed between posterior and anterior urethra is the membranous urethra, which explains why radiotherapy related strictures fall predominantly in this location. Urethral vascular anatomy informs reconstructive strategies in pediatric and adult urological surgery such as division of the urethral plate during anastomotic urethroplasty or chordee correction, reconstruction of failed hypospadias, staged and one stage substitution procedures, as well as microsurgical revascularization for urethral or erectile problems–beyond the scope of this chapter [7]. Analogously, the penile erectile bodies and glans can be split, separately mobilized, and even transected and reattached yet be expected to heal reliably in the absence of all but the most severe vascular disorders (such as Monckeberg’s calciphylaxis). The ultimate demonstration of the robustness of the penile and urethral vasculature is penile disassembly advocated in selected cases of complete bladder exstrophy and epispadias.
The skin of the external genitalia has a similar redundant blood supply. Penile shaft skin receives primary vascular support from the superficial external pudendal arteries with collateral flow from the bulbourethral and dorsal arteries via the glans. The scrotum receives arterial input from the named arteries off of deep external pudendal arteries as well as anastomoses with the perineal arteries posteriorly. Thus lacerations, burns, surgical incisions, and a variety of random flaps can reliably heal when based on genital skin. An exception to this rule of thumb is the perineum. If the skin is lost through necrotizing soft tissue infection, the underlying perineal soft tissue and fat poorly support skin grafts and often must be left to close by secondary intention or with the support of vascularized tissue transfer.
Tissue Healing Responses
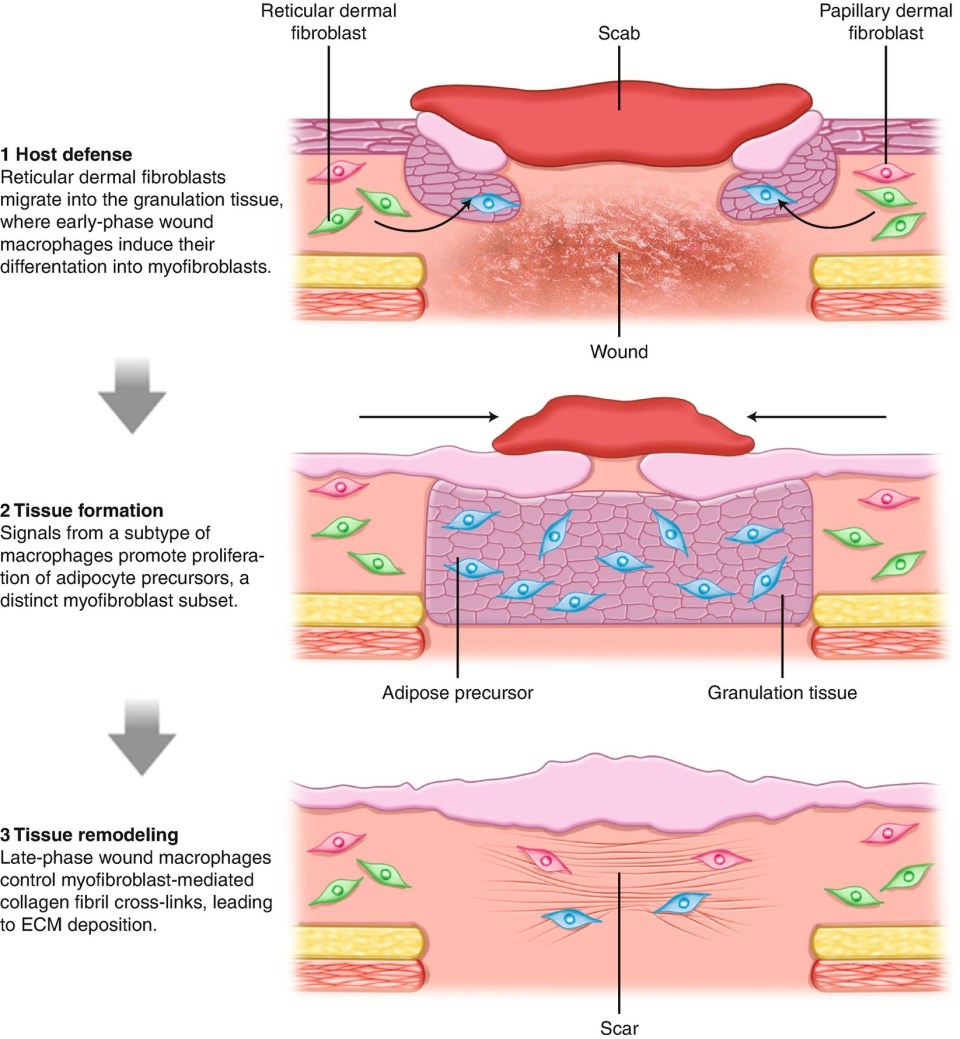
With rare exception of certain organs and fetal tissue, the healing response replaces damaged tissue through the deposition of collagenous connective tissue. Fibrosis to temporarily stabilize newly formed or newly connected tissues is important for healing, but may be perturbed by excessive fibrosis which ultimately impairs tissue function and leads to patient discomfort, loss of function, and even mortality.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







