and Christopher Isles2
(1)
Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK
(2)
Dumfries and Galloway Royal Infirmary, Dumfries, UK
Q1 Describe the different types of renal replacement therapy.
There are three. These are haemodialysis, peritoneal dialysis and transplantation. Haemodialysis may be undertaken in hospital or at home. Peritoneal dialysis is always done at home either during the day (Continuous Ambulatory Peritoneal Dialysis or CAPD) or overnight (Automated Peritoneal Dialysis or APD). Transplantation may either be by cadaveric or live donor. Conservative care is a further treatment option for patients with end stage renal disease and may well be the appropriate form of treatment for the frail elderly with multiple co-morbidities. PD, transplantation and conservative care are discussed in more detail in the chapters that follow.
Q2 How many patients receiving RRT will be on haemodialysis, peritoneal dialysis or have a functioning transplant?
The take on rate for renal replacement therapy appears to be plateauing at around 100 patients per million per year in the UK, though higher take on rates are seen in those parts of the country with significant Black African and Asian communities. Good pre-dialysis education is nowadays usually possible for all except those who present acutely, and means that patients are able to weigh up the pros and cons of each type of treatment and then make an informed decision. Latest UK figures from December 2012 suggest that despite efforts to promote PD, 86 % of dialysis patients (43 % of the total RRT population) will end up doing HD and only 14 % of dialysis patients (7 % of the total RRT population) will be on PD. Disappointingly very few haemodialysis patients choose or are prepared to dialyse at home (“home-haemo”) which means that home haemodialysis accounts for only 2 % of the total RRT population. The split between CAPD and APD among those doing peritoneal dialysis is such that around one third choose CAPD and two thirds choose APD (Fig. 37.1).
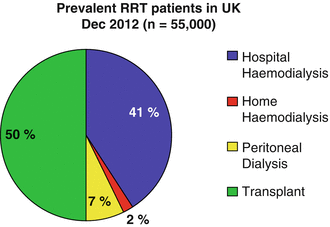

Fig. 37.1
UK prevalence by RRT modality UK Renal Registry, The Sixteenth Annual Report, 2013
Q3 How does haemodialysis work?
Haemodialysis was the first form of renal replacement therapy to be developed in the 1960s and remains the most common dialysis modality. Blood from the patient is pumped through a dialysis filter which removes waste products (urea) and fluid, before being returned to the patient. Urea moves from an area of high concentration to one of low concentration across a highly permeable dialysis membrane by a process known as diffusion. Fluid is removed by creating a negative transmembrane pressure in a process known as ultrafiltration. Successful haemodialysis requires good vascular access (see next question) (Fig. 37.2).
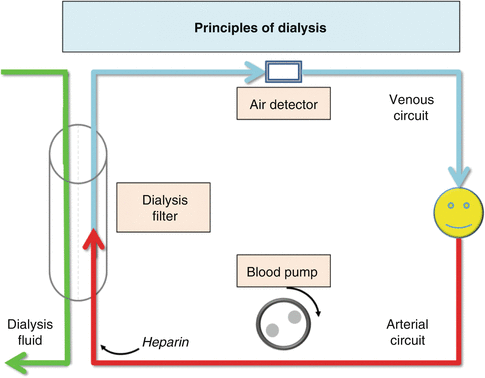

Fig. 37.2
A basic haemodialysis circuit
Q4 What is meant by the term vascular access?
Vascular access is the means by which patients are connected to their dialysis machines. Broadly speaking there are three types – fistulas, lines and grafts. An arteriovenous fistula is created by joining an artery to a vein in the wrist (radiocephalic fistula) or in the elbow (brachiocephalic fistula) under a local anaesthetic (Fig. 37.3). It is recommended that two thirds of patients should start haemodialysis with a fistula and that 80 % of all haemodialysis patients in each renal unit should have one. There are two types of line – temporary and tunnelled. Both are usually placed in the internal jugular vein under local anaesthetic. Temporary lines are fairly easy to insert and remove while tunnelled lines require considerably more expertise. The term ‘tunnelled’ refers to the subcutaneous tunnel leading to an exit site on the anterior chest wall which offers a greater degree of protection against infection than a temporary line. Grafts are the third form of access. They are created by joining arteries to veins by a short tube of Gore-tex. Currently less than 5 % of haemodialysis patients have one.
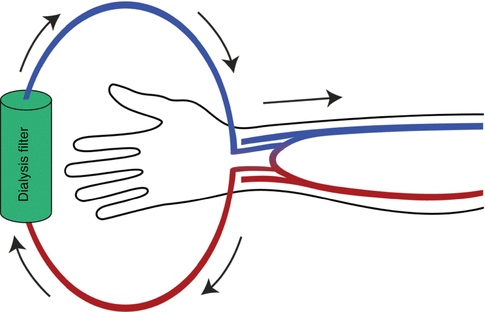

Fig. 37.3
An AV fistula sited in the forearm. Note blood leaving the arterial limb, passing through the dialyser and returning via the venous limb
Q5 When should vascular access be discussed with patients?
The question of vascular access should be discussed once a patient with progressive renal failure has considered all the options and expressed a preference for haemodialysis rather than PD. This discussion will generally take place when eGFR is around 20 ml/min and will usually lead to referral for a fistula. A fistula is the preferred vascular access for haemodialysis because of the lower risk of clot formation and infection provided by the use of native vessels. Once a decision to create a fistula has been taken everything should be done to preserve the forearm veins by avoiding venepuncture and cannula insertion whenever possible.
Q6 Describe what happens when a fistula is created
Joining an artery to a vein in the wrist or antecubital fossa means that blood is diverted up the vein at arterial pressure without having to pass through the capillary network first. Exposing a vein to blood in this way leads to distension and thickening (so called arterialisation) of its wall. Fistulas take 6–8 weeks to mature and should be ready to use at the time of first dialysis which means the best time to create a fistula is at least 6–8 weeks before it is needed. Fistulas in people with diabetes and in non-diabetics with small arteries or difficult veins may need longer to mature. Timing is everything.
Q7 How do you know when to start dialysis in a patient with CKD?
The mnemonic AEIOU gives the five clinical indications for starting dialysis:
Box 37.1 Reasons for starting dialysis
A
Acidosis eg if serum bicarbonate <10 mmol/l
E
Electrolytes eg when serum potassium >7 mmol/l
I
Inflammation i.e. pericarditis
O
Overload, meaning pulmonary oedema
U
Uraemia
The commonest reasons for starting dialysis are uraemia, fluid overload and hyperkalaemia. Uraemia, specifically “symptomatic uraemia”, refers to patients who have a persistently high level of blood urea and develop the symptoms of anorexia, nausea and vomiting. Acidosis is rarely the main reason for starting dialysis but is often present when the patient becomes uraemic. Pericarditis is not present in every case, but is certainly an indication to start dialysis if the characteristic rub is heard. In practice we aim to start dialysis just before the symptoms or signs become clinically significant i.e. dialysis should be initiated to maintain wellbeing rather than to rescue from illness.




Q8 How does eGFR help us decide when to start dialysis?< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








