 INTRODUCTION
INTRODUCTION
CKD is a worldwide health problem. Comprehensive data on CKD provided by the Third National Health and Nutrition Examination Survey (NHANES III) note that approximately 800,000 Americans have CKD as manifested by a serum creatinine concentration of 2 mg/dL or greater. More than 6.2 million are estimated to have a serum creatinine concentration of 1.5 mg/dL or greater. Data extrapolated from the Framingham study suggest that approximately 20 million people in the United States are at risk for CKD.
The rapid growth in both the incidence and prevalence of CKD will result in a huge influx of patients into the ESRD system. Based on data from the United States Renal Data System (USRDS), the number of point prevalent Medicare ESRD patients increased to more than 470,000 (3.2% difference) and that of the non-Medicare ESRD patients increased to 101,351 (8.3% difference) from 2008 to 2009. Expansion of the ESRD population will have a significant economic impact on the already overextended Medicare system. The per-person per-year costs (net inpatient/outpatient) attributed to CKD among Medicare patients is generally higher in those with later stages of CKD as compared to earlier stages. For instance, the costs were approximately $19,052 for Stages 4 to 5 CKD as compared with $13,120 for Stages 1 to 2 CKD (a 45% difference). These costs included medical and surgical diagnostic-related groups (DRGs), pharmacy supplies, home health agencies, and skilled nursing, among others. For ESRD, the total Medicare costs for 2009 rose to $29 billion, which accounts for 5.9% of the total allotted Medicare budget for that year. The increase in both CKD and ESRD populations may also overwhelm the ability of nephrologists and other healthcare providers to fully provide interventions that will improve the length and quality of patients’ lives.
Defining and Staging Chronic Kidney Disease
Several terms are used to describe the period of kidney disease that precedes the institution of RRT such as pre-ESRD, chronic renal insufficiency, chronic renal failure, and chronic renal disease. Unfortunately, none of these terms is particularly accurate and may be confusing to nonnephrology physicians. The term pre-ESRD gives the impression that dialysis is an inevitable outcome of all kidney diseases. The terms renal insufficiency, chronic renal failure, chronic renal disease, and pre-ESRD have negative connotations. These terms also include the word renal, which is not easily understood by patients. For these reasons, chronic kidney disease is chosen as the defining term.
The definition and classification of CKD are based on measurement of GFR, the best overall measure of kidney function. Factors that influence GFR include both structural or functional kidney disease, as well as patient age. In general, the annual decline of GFR with age is approximately 1 mL/min/1.73 m2 of body surface area, beginning after the patient reaches approximately 20 to 30 years of age. Although a chronic decline in GFR to a level of less than 60 mL/min/1.73 m2 is evidence of CKD, substantial kidney damage can exist without a decrease in GFR. In this circumstance, kidney damage is defined as a structural or functional abnormality of the kidney that persists for more than 3 months. Manifestations of kidney damage can include pathologic changes or abnormalities revealed by blood, imaging, or urine tests. Using this definition, CKD is present if the GFR is less than 60 mL/min/1.73 m2. CKD is also present if the GFR is equal to or greater than 60 mL/min/1.73 m2, if other evidence of kidney damage also exists. Table 16.1 provides a classification and staging system based on the level of GFR.
![]() TABLE 16-1. Staging System and Action Plan for Chronic Kidney Disease
TABLE 16-1. Staging System and Action Plan for Chronic Kidney Disease

Since the inception of the classification system in 2002, there have been a few modifications. In 2005, the Kidney Disease: Improving Global Outcomes (KDIGO) Work Group recommended adding the suffix “D” for patients with Stage 5 CKD who were on dialysis and the suffix “T” for those with a functioning kidney transplant. Three years later, the United Kingdom National Institute of Health and Clinical Excellence (NICE) group recommended subdividing Stage 3 CKD into 3a (GFR 59 to 45 mL/min/1.73 m2) and 3b (44 to 30 mL/min/1.73 m2) and adding the suffix “p” for those with confounding proteinuria. These modifications were based on the fact that a lower GFR in Stage 3 and the presence of proteinuria had significant implications on clinical outcomes.
This staging system provides a common language for communication between the various healthcare providers. It allows more reliable estimates of the prevalence of earlier stages and of populations at increased risk for CKD. In addition, evaluation of factors associated with a high risk of progression can be recognized. Treatments can be more effectively examined and the development of adverse outcomes in this population is more easily determined.
Glomerular Filtration Rate as an Index of Kidney Function
Serum creatinine concentration is commonly employed as an index of renal function. It is not an accurate measure of GFR, however, and it is especially inaccurate when the serum creatinine concentration is between 1 and 2 mg/dL. This is because creatinine, unlike inulin, is secreted by the renal tubules. As renal function declines, the amount of creatinine secreted by the tubules increases and raises the amount of creatinine in the urine. This acts to falsely increase the creatinine clearance (CrCl), resulting in an overestimation of GFR. Serum creatinine concentration is also influenced by body mass, muscle mass, diet, drugs, and laboratory analytical methods. “Normal” ranges of serum creatinine quoted by laboratories are misleading because they do not take into account the age, race, sex, or body size of the individual.
Inulin clearance is the gold standard test for measuring GFR. Unfortunately, this test is cumbersome, expensive, and not widely available for clinical use. Iothalamate (125I-iothalamate) clearance estimates GFR and is a reasonably accurate substitute for the inulin clearance method. It is also expensive and somewhat cumbersome to perform as a routine clinical test. A 24-hour urine collection for CrCl is the accepted alternative measure of GFR because it is widely available and is familiar to most clinicians. It is often difficult, however, for patients to perform correctly and is less accurate than either inulin or iothalamate clearance. In addition, this test often overestimates GFR in patients with advanced kidney disease.
To simplify measurement of renal function, GFR estimates from prediction equations are often used. These formulas take into account serum creatinine concentration, age, gender, race, and body size, and are better estimates of GFR than serum creatinine concentration alone. The formulas used are sufficiently accurate. The three most widely used are the Cockcroft-Gault, the Modification of Diet in Renal Disease Study (MDRD), and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations. The Cockcroft-Gault equation noted below estimates of creatinine clearance (eCrCl):
![]()
Although it provides an adequate estimate of GFR (eGFR), the MDRD equations are more accurate. MDRD equation 7 is the preferred formula but it requires measurement of blood urea nitrogen (BUN) and serum albumin. The MDRD formula is as follows:
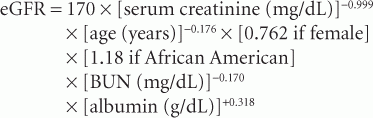
An abbreviated form of the MDRD equation that does not require BUN or albumin measurement was also developed and is as follows:

The abbreviated form is reasonably accurate. The MDRD equation was tested in more than 500 patients with a range of kidney diseases and ethnicities (European Americans and African Americans). GFR values were validated in the sample group using 125I-iothalamate as the gold standard; however, certain patient groups were not well represented in the MDRD study sample. Therefore, clearance measurements are still required in groups who were underrepresented in the MDRD sample to fully validate the formula for all patients. These include patients at extremes of age and body size; the severely malnourished or obese; patients with skeletal muscle diseases, paraplegia or quadriplegia; vegetarians; and those with rapidly changing kidney function. The MDRD equation underestimates GFR in patients with relatively normal kidney function.
In 2009, the CKD-EPI was developed in an attempt to improve the accuracy of estimating equations in a more heterogenous group of patients. This formula utilized the same 4 variables as the MDRD equation. Compared with the MDRD equation, the CKD-EPI equation has less bias, particularly at GFR greater than 60 mL/min/1.73 m2, as well as improved overall accuracy. It also allows reporting of numeric values across the range of GFR measurements. The CKD-EPI equation is as follows:

k is 0.7 for females and 0.9 for males, a is –0.329 for females and –0.411 for males.
In the absence of specific modifications for race, ethnicity or regional difference, the CKD-EPI equation is fairly accurate for GFR estimation. To account for possible differences in muscle mass and diet, race, ethnicity and other geographic variables, the MDRD Study and CKDEPI equations have been modified for use in China and Japan. The modifications are associated with improved accuracy in these populations.
It has been proposed that utilizing both serum creatinine concentration and serum cystatin C together may improve the accuracy of GFR estimation, as compared to either marker alone. This may be particularly useful in patients with CKD 3A (45 to 59 mL/min/1.73 m2) who do not manifest any markers of kidney damage. Cystatin C may potentially offer some advantages over measurement of serum creatinine concentration in the estimation of GFR and for the proper classification of CKD. Its use, however, is limited by higher cost and “lack of standardization” among the limited number of laboratories that offer the test.
Prevalence of Chronic Kidney Disease Stages
Prevalence estimates for each CKD stage were obtained by using a reference group comprised of patients evaluated in the NHANES III. In this sample of patients, the MDRD equation was used to estimate GFR. In addition to abnormal GFR levels, the presence of micro- or macroalbuminuria on spot urine specimens was considered sufficient evidence of kidney damage. The level of albuminuria, based on the ratio of albumin (and protein) to creatinine on spot urine samples, was used to estimate the prevalence of the first 2 stages. The reported prevalence of CKD Stages 1 to 4 in the most recent NHANES between 1999 and 2006 was 26 million (13%) out of approximately 200 million United States residents 20 years of age or older. Approximately 65% had CKD Stage 3 or 4. The USRDS estimates that nearly one-half million U.S. patients were treated for ESRD in the year 2004, and by 2010 this figure increased by approximately 40%. The elderly are a growing segment of the population and are clearly at increased risk for kidney disease. Males and African Americans with preexisting hypertension or diabetes mellitus and CKD are also at higher risk for development of ESRD.
 APPROACH TO CHRONIC KIDNEY DISEASE PATIENTS
APPROACH TO CHRONIC KIDNEY DISEASE PATIENTS
The approach to the patient involves establishing the presence of CKD, determining the stage of disease, and enacting an action plan based on the stage. The management of CKD patients requires a multidisciplinary approach involving primary care physicians, nephrologists, endocrinologists, cardiologists, vascular surgeons, physician assistants, nurse practitioners, dietitians, and social workers. The goals of this interdisciplinary approach are to identify patients either with or at increased risk for CKD, to slow the progression of CKD to ESRD, to identify and treat comorbid conditions, to identify and prevent complications of CKD, and to prepare patients mentally and physically for RRT. As seen in Table 16.1, the action taken increases from simple screening maneuvers and risk reduction to more complex disease management.
Patients with established CKD are assessed for comorbid conditions. Medications are adjusted for the level of renal function. Blood pressure (BP) monitoring is essential to diagnose hypertension and facilitate optimal BP control. Serum creatinine concentration is measured to allow estimation of GFR. Protein- or albumin-to-creatinine ratios on spot urine samples and urinalysis are performed. Finally, imaging of the kidney by ultrasound is warranted in most CKD patients.
The approach is implemented in a stepwise fashion and individualized for each patient based on the level of kidney function. In a patient with a normal GFR (≥90 mL/min/1.73 m2 ) or a mildly impaired GFR (>60 mL/min/1.73 m2) the focus will be on delaying progression and treating comorbid conditions. Progression is best predicted by plotting the reciprocal of the serum creatinine concentration over time. This plot predicts a date when the GFR will reach target levels and can be used along with symptoms and signs for deciding the appropriate time for initiation of RRT.
KEY POINTS
 PROGRESSION OF CHRONIC KIDNEY DISEASE
PROGRESSION OF CHRONIC KIDNEY DISEASE
Mechanisms of Chronic Kidney Disease Progression
Much of what we understand about the mechanisms involved in the progression of CKD has been obtained through “experimental kidney disease.” Progression of CKD may be considered as a process of “glomerular adaptation.” In experimental models, adaptation is characterized by an increased workload per nephron, and this is manifested as increased “single nephron GFR (SNGFR).” The increase in SNGFR is initially “adaptive,” but eventually becomes “maladaptive,” because it leads to further nephron injury. There are several theories that have been suggested to account for this:
1. Hemodynamic hypothesis
2. Abnormal permeability to macromolecules
3. Growth Factor Hypothesis
Hemodynamic Hypothesis
In experimental settings, ablation of kidney mass is achieved through a unilateral nephrectomy followed by ligation of the renal artery branches in the remaining functioning kidney, thereby causing an infarction of approximately two-thirds of said kidney. By reducing the number of nephrons to one-sixth, GFR reduction ensues. Following a reduction in the number of functioning nephrons, the remaining nephrons experience hyperfiltration and glomerular capillary hypertension. Although these changes are initially adaptive to maintain GFR, over time they are deleterious to renal function because of pressure-induced capillary stretch and glomerular injury. Histopathologically, this progression of events is manifested as glomerular and tubular hypertrophy followed by eventual focal glomerular sclerosis, tubular atrophy, and interstitial fibrosis. Damage caused by glomerular hyperfiltration is notably important in the pathophysiology that underlies diabetic nephropathy.
Another experimental kidney disease model mimicking diabetes mellitus utilizes alloxan or streptozotocin to chemically ablate pancreatic islet cells. The hyperfiltering state induced by hyperglycemia upregulates local expression of the renin-angiotensin-aldosterone system (RAAS) and contributes to progressive kidney damage. In this instance, stimulation of the RAAS causes glomerular injury by further raising glomerular capillary pressure through angiotensin II (AII)-driven efferent arteriolar vasoconstriction and facilitating pressure and stretch injury in the capillaries. Taken together, these effects lead to endothelial injury, stimulation of profibrotic cytokines by the mesangium, and detachment of glomerular epithelial cells.
Abnormal Permeability to Macromolecules
Another consequence of renal injury and activation of the RAAS is proteinuria. Glomerular capillary hypertension, caused by hyperfiltration and AII effect on efferent arterioles, leads to an increase in glomerular permeability and excessive protein filtration. Pore size is altered by AII, increasing protein leak across the glomerular basement membrane. An activated RAAS may also cause proteinuria through novel effects on nephrin expression in kidney. Nephrin, a transmembrane protein located in the slit diaphragm of the glomerular podocyte, is thought to play a key role in the function of the glomerular filtration barrier. By maintaining slit diaphragm integrity, nephrin limits protein loss across the glomerular basement membrane. When its expression is disrupted, proteinuria and its consequences may result. Data in rat models of proteinuric kidney disease suggest an important interaction between the RAAS and nephrin in modifying glomerular protein permeability. Although proteinuria is a marker for renal disease risk, it is also likely that excess protein in urine contributes to progressive kidney damage. Proteins present in the urine are toxic to the tubules, and can result in tubular injury, tubulointerstitial inflammation, and scarring. Tubular damage is caused by protein overloading of intracellular lysosomes, stimulation of inflammatory cytokine expression, and extracellular matrix protein production. These processes induce renal tubulointerstitial fibrosis and glomerular scarring. Remission or reduction in proteinuria is often associated with renoprotection and slowed progression of kidney disease.
Growth Factor Hypothesis
Although it is known that elevated glomerular capillary pressure and capillary stretch lead to scar formation in the glomerulus, an activated RAAS and other inflammatory mediators cause irreversible damage in the kidney through other mechanisms. Proinflammatory and profibrotic effects of AII and aldosterone underlie the injury that develops in the renal parenchyma.
Advanced glycation end-products (AGEs) accumulate in the mesangial area and glomerular capillary walls in diabetic nephropathy patients, and as such may have a role in perpetuating renal injury. AGEs are a heterogeneous group of compounds that are produced by nonenzymatic, sequential glycation and oxidation reactions of sugars with free amino groups on proteins, peptides, or amino acids. There are several pathways by which AGEs cause renal injury:
1. AGEs interfere with extracellular matrix proteins (collagen, elastin, and laminin) leading to alterations in both structure (induces fibrosis) and function (hydrophobicity, charge, elasticity, and turnover).
2. AGE–RAGE interactions. AGE may also produce cellular injury by a cascade of receptor-dependent (RAGE) events that leads to transformation of tubular cells into myofibroblasts, leading to development of tubular atrophy and interstitial fibrosis.
3. AGEs are also involved in receptor-independent interactions that lead to intracellular generation of reactive oxygen species (ROS). ROS activate signaling pathways (eg, mitogen-activated protein kinases, protein kinase C, Janus kinase/signal transducers and activators of transcription), which lead to proinflammatory (eg, nuclear factor kappa B [NF-κB], monocyte chemoattractant protein-1, tumor necrosis factor [TNF]-α) and profibrotic (eg, transforming growth factor [TGF]-β, connective tissue growth factor, platelet-derived growth factor [PDGF]) effects.
4. Accumulation of AGEs also leads to endothelial dysfunction (indirectly), increased thrombogenicity and accelerated atherosclerotic changes, and subsequent end-organ hypoperfusion.
Another maladaptive consequence is increased ammoniagenesis per remnant nephron. This effect promotes complement cascade activation and enhanced injury to the tubulointerstitium. These effects are thought to be related to the actions of excess aldosterone and endothelin-1 stimulated by impaired elimination of the daily acid load and subsequent acid retention (inherent in CKD). This concept has led to the notion that dietary alkali therapy may have a potential role in preserving GFR and delaying progression of CKD.
These various mediators promote fibrosis and scarring in the kidney through multiple untoward effects such as toxic radical formation, enhanced cellular proliferation, and collagen deposition in the glomerulus and tubulointerstitium. Ultimately, glomerulosclerosis and tubulointerstitial fibrosis occur and promote CKD.
 RISK FACTORS FOR PROGRESSION OF CKD
RISK FACTORS FOR PROGRESSION OF CKD
The risk factors for CKD progression can be classified into (Table 16.2):
1. Susceptibility factors—These are the factors that predispose to CKD. These include genetic and familial predispositions, race, maternal-fetal factors, age, and gender.
2. Initiation factors—These are the factors that precipitate injury to the kidneys.
3. Progression factors—These are the factors associated with progression of damage to established kidney disease.
![]() TABLE 16-2. Risk Factors associated with Initiation and Progression of Chronic Kidney Disease
TABLE 16-2. Risk Factors associated with Initiation and Progression of Chronic Kidney Disease

These factors are further classified as either modifiable or nonmodifiable, based on feasibility for intervention. Below are the modifiable risk factors for progression.
Hypertension and the RAAS
Hypertension is clearly associated with progression of CKD and is the second most common cause of ESRD. Importantly, hypertension is present in the majority of CKD patients, making it a key risk factor for progression. Most studies, with a few exceptions confirm that hypertension hastens the course of CKD to ESRD in both diabetic and nondiabetic patients. The MDRD study demonstrated that proteinuric patients, when randomized to a lower BP, manifested a slower decline in GFR. Also, significant correlation between the achieved BP and the rate of renal function decline, especially in patients with greater than 1 g/day of proteinuria, was noted. The Joint National Committee (JNC VII) recommends the following BP target goals:
1. CKD with less than 1 g/day of proteinuria: 130/80 mmHg.
2. CKD with more than 1 g/day of proteinuria: 125/75 mmHg.
Since the JNC VII Guidelines were published in 2003, several studies have questioned the recommendation of targeting a BP of 130/80 mmHg in CKD patients without albuminuria. Studies suggest that data from the general population are not necessarily applicable to the CKD population. Furthermore, some suggest that tight BP control may have adverse consequences particularly in the elderly and those with coronary artery disease. Several randomized controlled trials (RCTs) failed to demonstrate a significant benefit of aggressive lowering of in those without proteinuria. The AASK (African American Study of Kidney Disease and Hypertension) found there was notable benefit in targeting a lower BP (mean arterial pressure [MAP] ≤92 mmHg) for those with urine protein-to-creatinine ratio greater than 220 mg/g, whereas, no benefit was seen in those with urine protein-to-creatinine ratio less than 220 mg/g. A similar finding was demonstrated in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, whereby a target systolic BP less than 120 mmHg was not significantly beneficial, as opposed to a target systolic BP less than 140 mmHg. Analysis of the effect of BP control on progression to ESRD was assessed in 16,128 CKD patients in the Kidney Early Evaluation Program (KEEP). In this large, diverse population, progression to ESRD started at a systolic BP of 140 mmHg rather than the recommended goal of 130 mmHg. Progression was highest in those with a systolic BP at least 150 mmHg. Thus, we may need to change target BP for CKD patients.
Proteinuria is a powerful risk factor for progression of CKD, especially as levels exceed both 1 and 3 g/day, respectively. Patients with high-grade proteinuria and hypertension are at highest risk to progress to ESRD. MDRD Study A data demonstrated significant benefit in kidney outcomes in patients with proteinuria greater than 1 g/day, particularly in those with GFR between 25 and 55 mL/min/1.73 m2 and a trend toward benefit in patients with lower levels of proteinuria. This was supported by the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) study, which also showed that a low BP target was beneficial in decreasing the risk of kidney outcomes in those with higher urine protein levels.
Both experimental and clinical data suggest that inhibition of the RAAS is very effective in lowering BP, reducing proteinuria, and slowing progression of kidney disease in both diabetic and nondiabetic patients. This is of particular interest as the leading cause of ESRD in the United States is diabetic nephropathy. Treatment of disease states resulting from or associated with excessive RAAS activity is best achieved by therapies that suppress AII and aldosterone production or inhibit the renal effects of these substances (Figure 16.1).

FIGURE 16-1. The RAAS. AII and aldosterone are formed by classical pathways (renin, angiotensin-converting enzyme [ACE]) and alternate pathways (tonin, tPA, cathepsin G, chymase, CAGE). The pathway is interrupted at various levels by ACE inhibitors, AT1 receptor antagonists, and aldosterone receptor antagonists. Abbreviations: AT1, angiotensin type 1; AT2, angiotensin type 2; CAGE, chymostatin-sensitive angiotensin II-generating enzyme; tPA, tissue plasminogen activator. (Courtesy of Mark A. Perazella.)
Inhibition of angiotensin-converting enzyme (ACE) activity decreases AII and aldosterone formation and potentiates the vasodilatory effects of the kallikrein-kinin system by increasing bradykinin formation (Figure 16.1). The ACE inhibitors reduce proteinuria and delay progression of kidney disease in both diabetic nephropathy and other forms of proteinuric kidney disease. In a landmark study, the effect of captopril versus conventional therapy on the occurrence of multiple renal end points (time to doubling of serum creatinine concentration, progression to ESRD, or death) was studied in 409 type 1 diabetic patients with proteinuria and CKD. A 50% reduction in the development of these renal end points was demonstrated in patients treated with captopril compared with conventional therapy, despite little difference in BP control. The beneficial effects of RAAS inhibition also extend to nondiabetic kidney diseases complicated by proteinuria. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study compared the ACE-inhibitor benazepril with placebo in 583 nondiabetic patients with CKD. Benazepril was associated with an overall risk reduction of 53% in the development of the primary renal end point (doubling of serum creatinine concentration and need for dialysis) as compared with conventional antihypertensive therapy. In this trial, the absolute benefit of ACE inhibition was most marked in patients with the highest level of proteinuria. The Ramipril Efficiency in Nephropathy (REIN) study (stratum 2) confirmed these positive results in a similar group of nondiabetic patients. A 52% risk reduction in progression to kidney disease end points was seen with ramipril as compared with placebo. Renoprotection was most impressive in patients with greater than 3 g of proteinuria. A meta-analysis of data obtained from 1860 nondiabetic patients from 11 randomized clinical trials demonstrated significant renal protection with ACE inhibitors. ACE-inhibitor therapy was associated with a reduction in relative risk for the development of ESRD (0.69) and for the doubling of serum creatinine concentration (0.70). Thus, the benefit of ACE inhibition is most pronounced in patients with heavy proteinuria and a reduction in proteinuria correlates with slower declines in GFR.
AII type 1 receptor blockers (ARBs) lower BP, reduce proteinuria, and slow progression of kidney disease. Antagonism of the AT1 receptor (see Figure 16.1) and binding of AII to the AT2 receptor probably underlies their mechanism of action. Recently completed clinical trials suggest that ARBs reduce microalbuminuria and proteinuria and retard the progression of diabetic CKD in a fashion similar to the ACE inhibitors. The Reduction of End Points in NIDDM with the Angiotensin II Receptor Antagonist Losartan (RENAAL) study compared the ARB losartan with conventional therapy in 1513 type 2 diabetics with hypertension and nephropathy. A 16% risk reduction was noted in predetermined primary composite end points (time to doubling of serum creatinine concentration, progression to ESRD, or death) in the losartan group over a mean follow-up of 3.4 years. This study demonstrated a 28% risk reduction in progression to ESRD and 25% reduction in doubling of serum creatinine concentration in patients treated with losartan. An average reduction in the level of proteinuria of 35%, despite similar BP control between the groups, was also noted. Similar findings were described in the Irbesartan Diabetic Nephropathy Trial (IDNT) study, which employed irbesartan in patients with type 2 diabetes mellitus and nephropathy. The Telmisartan Randomized Assessment Study in ACE-intolerant Subjects with Cardiovascular Disease (TRANSCEND) study, which included patients with vascular diseases and diabetes, showed that telmisartan significantly decreased the risk of composite kidney outcomes (as compared to placebo) in those with microalbuminuria (defined as urine microalbumin-to-creatinine ratio >3.4 mg/mol).
Like ACE inhibitors, interruption of the RAAS with ARBs in diabetics is a logical, albeit incomplete, strategy to provide renoprotection. In addition, both ACE inhibitors and ARBs are also associated with so-called off-target effects, which are not related to RAAS inhibition. For instance, the ARB losartan has the unique ability to increase urinary uric acid excretion. Other effects attributed to ACE inhibitors and ARBs include decreases in hemoglobin and serum cholesterol levels (especially in proteinuric subjects).
The choice between ACE inhibitors and ARBs in CKD, however, is an area of controversy. In general, the evidence for ACE inhibitors and improved kidney outcomes are older and mostly apply to type 1 diabetics. On the other hand, evidence for the use of ARBs in type 2 diabetics is contemporary. Data on cardiovascular protection in diabetic patients are noted with ACE inhibitors. Current evidence suggests that the effects of both agents are likely similar. A recent metaanalysis noted that there was insufficient evidence on the relative effects on survival when comparing both classes. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Randomized Assessment Trial (ONTARGET), which enrolled people with high cardiovascular risk (including those with diabetes and CKD), did not show a clear difference between the 2 classes of drugs. This study, however, was believed to be relatively underpowered for this comparison.
Previously, dual blockade of the RAAS with ACE inhibitors and angiotensin receptor blockers was considered to provide kidney benefit beyond therapy with either drug alone. One notable study that supported this notion was the Candesartan and Lisinopril Microalbuminuria (CALM) study. This study combined lisinopril and candesartan to treat hypertension and reduce microalbuminuria in patients with type 2 diabetes mellitus. Over 24 weeks, dual blockade safely reduced BP and reduced microalbuminuria (50%) as compared with candesartan (24%) and lisinopril (39%) monotherapy. Similarly, a randomized double-blind crossover study in 18 type 2 diabetic patients with proteinuria demonstrated positive renal effects with combination therapy. In patients with immunoglobulin (Ig) A nephropathy, the combination of losartan and enalapril were additive in decreasing urinary protein excretion, whereas doubling the dose of either form of monotherapy had no effect on proteinuria. Over 6 months, the combination of lisinopril plus candesartan reduced proteinuria by 70% compared to monotherapy with lisinopril (50% reduction) or candesartan (48% reduction). Not all studies demonstrate that combination therapy is better than maximal dose ACE-inhibitor therapy in decreasing proteinuria. These studies suffer from small patient numbers, surrogate markers of renal protection (proteinuria), and short-term follow-up.
In a recent trial, combination RAAS blockade therapy was associated with an increase in adverse events, especially impaired kidney function and hyperkalemia, as compared with either agent alone. This occurred despite significant albuminuria reduction with combination therapy. In ONTARGET, combination therapy failed to improve cardiovascular end points despite additional BP reduction averaging systolic blood pressure 2.4 mmHg/diastolic blood pressure 1.4 mmHg. At the present time, dual RAAS blockade with an ACE inhibitor and an ARB is not recommended, a recommendation supported by the American Society of Hypertension’s Position Article on combination therapies. Thus, titration of the single agent to maximal dose to control BP and proteinuria is recommended. If proteinuria remains greater than 1 g/day, a second agent to further block the RAAS is not recommended but may be useful in certain individuals. The risks and benefits of this therapy must be carefully weighed.
Aldosterone, the last hormone in the RAAS pathway is associated with renal injury through both hemodynamic and profibrotic effects. Aldosterone antagonism in animals is renoprotective when used alone or in combination with ACE inhibition. Preliminary human data suggest that the combination of an aldosterone receptor antagonist like spironolactone or eplerenone with an ACE inhibitor or ARB significantly reduce proteinuria. This therapy, however, is associated with higher risk of hyperkalemia.
Another class of drugs that act on the RAAS are the direct renin inhibitors (DRIs). Aliskiren, the first orally active DRI, decreases albuminuria when used in combination with losartan, in diabetic patients with proteinuria. This study also showed a near-significant trend toward a reduced GFR decline. However, a phase 3 study on type 2 diabetics with CKD combining aliskiren and another RAAS blocker, the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) study, was terminated because of futility and an increased incidence of stroke and serious adverse events (hyperkalemia, hypotension, and ESRD or death as a consequence of CKD). At present, aliskiren is not recommended for diabetic patients, particularly in combination with ACE inhibitors or ARBs.
Finally, it is important to recognize that with close patient monitoring, RAAS inhibitors can be used safely in most patients with mild-to-moderate CKD. The 2 major concerns associated with these drugs are the development of hyperkalemia and/or further worsening of kidney function. In regards to hyperkalemia, careful dose titration, dietary changes, avoidance of potassium-altering medications (nonsteroidal antiinflammatory drugs [NSAIDs], cyclooxygenase [COX]-2 selective inhibitors, potassium-sparing diuretics, etc), and use of loop diuretics allow safe therapy in most patients. Increases in serum creatinine concentration should be tolerated as long as the concentration rises no higher than 30% above baseline and stabilizes within 2 months of therapy. Continued increases should promote drug discontinuation and a search for volume contraction, critical renal artery stenosis, and other potentially correctable problems.
Diabetes Mellitus
As the prevalence of diabetes mellitus grows in the United States, patients with this disease continue to contribute a significant number of patients to the CKD population. In fact, diabetic kidney disease is the most common cause of ESRD. Thus, it is important to identify and adequately manage these patients to reduce progression of their underlying kidney disease. As shown in the Diabetes Control and Complications Trial (DCCT), intensive insulin therapy to establish tight glucose control prevented de novo kidney disease (microalbuminuria) by 34% and reduced progression of established nephropathy (albuminuria) by 56% in type 1 diabetics. Progression of CKD in type 2 diabetics is an even bigger problem as this group makes up the majority of patients who develop ESRD. Earlier studies revealed that intensive insulin therapy to maintain the glycosylated hemoglobin (HbA1c) level in the 7.0% to 7.6% range reduces progression of kidney disease (albuminuria/proteinuria) as compared with conventional insulin therapy.
Several trials conducted in diabetic patients to determine whether or not early and/or more intensive glycemic therapy might further decrease the frequency of CKD and ESRD. Aggressive glycemic control did not translate into better outcomes, and in certain situations, were harmful. Based on these trials, recommendations have been modified to target HbA1c approximately 7% to prevent or delay microvascular complications including overt diabetic nephropathy. Thus it appears that in diabetics with higher CKD stages, either high (>8% to 9%) or low (<7%) HbA1c levels are harmful in regards to mortality, progression of kidney disease and other clinical endpoints.
As HbA1c may not be truly accurate (falsely low as a result of decreased red blood cell [RBC] lifespan, transfusions, and hemolysis) in CKD patients, studies must adjust for this finding or develop another assay for these patients. To address this issue, research efforts are focused on glycated albumin as a measure of diabetic control in those with advanced stages of CKD.
Dietary Protein
Restriction of dietary protein reduces renal injury in the experimental setting by decreasing glomerular capillary hypertension and reducing production of profibrotic cytokines and growth factors. In humans, it is less clear that a low-protein diet is beneficial. The results of various studies are mixed. In the largest study, 2 levels of protein restriction (low and very low) failed to show a difference in GFR decline between groups after a mean follow-up of 2.2 years. Post hoc analysis identified some benefit of protein restriction when examined by achieved level of protein intake. Patients with the very low protein intake had a 1.15 mL/min/year slower decline in GFR. Two metaanalyses also suggest a benefit with protein restriction. In one, the risk of ESRD or death was reduced by 33% while another noted a small benefit in GFR change (0.53 mL/min/year) with a low-protein diet. Enthusiasm for this approach is tempered by the real risk of malnutrition in CKD patients.
Protein diets above the recommended daily intake may increase the rate of progression of kidney disease particularly in those with earlier stages of CKD. In the Nurses Health Study, the effect of protein intake over 11 years in 1624 enrolled females, divided into those with baseline GFR greater than 80 mL/min/1.73 m2 (normal kidney function) and those with baseline GFR 55 to 80 mL/min/1.73 m2, was examined. In those with normal baseline kidney function, there was no significant association between high protein intake and change in eGFR. However, in the latter group, protein intake was associated with a significant decrease in eGFR of approximately 1.69 mL/min/1.73 m2 per 10-g increase in protein intake. This effect was most significant in those who consumed a diet consisting of high nondairy animal-protein content.
Current evidence supports no benefit to dietary protein restriction of less than 0.8 g/kg/day. However, high total protein intake (>1.3 g/kg/day), especially high nondairy animal-protein content, may increase the rate of GFR decline in CKD patients, and is therefore not recommended.
Hyperlipidemia
Experimental work demonstrates that low-density lipoprotein (LDL) lipids are toxic to human mesangial cells, an effect that is reversed by 3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) reductase inhibitors (statins). Observational studies in humans suggest that reducing serum lipid levels is associated with preservation of kidney function. Unfortunately, these studies are plagued by small patient numbers and as a result, are underpowered for drawing any conclusions. To address this problem, a metaanalysis of 13 studies revealed a trend toward reduction in proteinuria and a small decrease rate of GFR loss with lipid lowering.
Two large scale RCTs (Prevention of Renal and Vascular End-stage Disease Intervention Trial [PREVEND-IT] and European Study for Preventing by Lipid-lowering Agents aNd ACE-inhibition Dialysis Endpoints (ESPLANADE) failed to show a beneficial effect of statins on albuminuria in patients who were treated with ARBs. The SHARP (Study of Heart and Renal Protection) study, a randomized, prospective, controlled trial examining the combination of ezetimibe and simvastatin, showed that all-cause and cardiovascular mortality were not improved. Combination therapy did not decrease the risk of progression to ESRD in CKD patients who were not on dialysis at baseline, as compared with placebo. The primary mortality benefit of therapy was limited to patients with hyperlipidemia, particularly CKD Stages 3 and 4 (but not in CKD Stage 5 or those on dialysis). Based on the results of SHARP, it appears that statin therapy does have a role—perhaps at least a statin combined with ezetimibe—in patients with CKD Stages 3 to 4. A major limitation is high cost.
Dietary Salt
CKD patients are at risk to develop salt and water overload as a consequence of reduced GFR, upregulated neurohormones, and other disturbed physiology. A direct correlation between a high sodium diet and increased arterial pressure, proteinuria, and decreased in GFR are well described.
A low-sodium diet may significantly reduce proteinuria and arterial pressure as shown in a crossover RCT where the addition of a low sodium diet to ACE inhibitor therapy significantly reduced proteinuria as compared with the addition of an ARB to ACE inhibitor therapy. Greater BP reduction was also noted with this approach. Current evidence supports lowering salt intake to less than 100 mmol (<2.4 g) per day of Na+ to achieve these clinical end points.
Hyperuricemia
CKD patients often develop hyperuricemia and/or gout from their reduced GFR, diuretics, and other abnormalities. An association between hyperuricemia in the setting of CKD and both negative cardiovascular outcomes progression of CKD is noted.
Reduction of symptomatic or asymptomatic hyperuricemia with the use of xanthine oxidase inhibitors, such as allopurinol, may slow loss of kidney function in CKD patients with and without diabetes mellitus. In one study, this effect was independent of other risk markers, for example, albuminuria. Other uric acid-lowering drugs, such as rasburicase and losartan, are associated with improved outcomes in CKD. In an 8-week study comparing rasburicase and placebo, a single 4.5-mg dose of rasburicase significantly lowered serum uric acid levels and improved kidney function. The ARB losartan, by virtue of its ability to increase urinary excretion of uric acid, lowered serum uric acid levels and reduced doubling of serum creatinine or development of ESRD.
Smoking
Tobacco smoking may injure the kidney through various pathways. Hypertension complicates smoking, a well-known factor associated with kidney disease. Smoking also increases SNGFR and may promote progression of kidney disease through hyperfiltration and glomerular capillary hypertension. Finally, smoking raises aldosterone levels. As discussed previously, aldosterone may enhance kidney disease by increasing BP and direct pro-fibrotic effects. In humans, smoking similarly injures the kidney and increases the risk of developing albuminuria in diabetics. Smoking cessation slows progression of kidney disease in patients with diabetic nephropathy and some nondiabetic forms of kidney disease. Given the overall negative health consequences associated with smoking, patients with CKD should be aggressively counseled to quit.
Obesity
Obesity is considered an independent risk factor for CKD. A metaanalysis of weight loss interventions in obese CKD patients showed an association between weight loss and a decrease in both proteinuria and systemic arterial pressure, with no demonstrable decrease in GFR during a mean follow up of 7.4 months. Weight loss interventions were shown to decrease proteinuria and albuminuria by 1.7 g and 14 mg, respectively. These effects were independent of BP reduction.
KEY POINTS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






