Gracilis Muscle Flaps
Oded Zmora
Fabio M. Potenti
Introduction
Reconstructive surgery of the perineal area, including complex perianal fistula management, reconstruction of the anal sphincter, and repair of complicated perineal wounds, is among the most challenging of tasks in colorectal surgery. These operations are associated with a substantial failure rate, and have a significant impact on patients’ quality of life.
Fistulas between the rectum and adjacent organs, such as the vagina or urethra, are among the toughest to treat, for several reasons. In many cases, the etiology of these fistulas includes conditions negatively affecting wound healing, such as history of radiation therapy (1,2), or inflammatory bowel disease (3). Occasionally, rectourethral fistulas may result from congenital malformations, which may be associated with other deformities of the perineal area. Anatomically, the rectum is adjacent to the vagina or the urethra, with only thin septum separating in between, which may further complicate attempts at local repair. In many cases, the rectal opening of such fistulas is situated in a proximal location above the dentate line, which makes transanal approach more complex.
Nonhealing perineal wounds result from previous surgery at the perineal area, such as abdominoperineal resection. Nonhealing wounds are more frequent following surgery for inflammatory bowel disease, or history of radiation therapy, and usually involve chronic tissue infection.
The gracilis muscle, located at the inner portion of the thigh, originates from the lower aspect of the anterior part of the pelvis and passes adjacent to the groin, throughout the inner part of the thigh, toward the knee. Its main innervation and blood supply enter the muscle proximally, near the groin, making it an ideal candidate for rotational flap during reconstructive surgery of the perianal area. The belly of the muscle can be rotated and inserted as a flap between the rectum and adjacent organs during the repair of fistulas, or used as filler for the repair of nonhealing perineal wounds, with the option of harvesting an island of skin from the medial aspect of the thigh as a myocutaneous flap. The gracilis muscle may also be used for reconstruction of the anal sphincter, as discussed in a separate chapter of this book.
Anatomy and Function of the Gracilis Muscle
The gracilis is the most superficial muscle on the medial aspect of the thigh. It is thin and flattened, broader at its base, gradually narrow, and tapering below. It arises from the anterior margins of the lower half of the symphysis pubis and the upper half of the pubic arch, runs vertically downward, ending in a rounded tendon. This tendon passes behind the medial condyle of the femur, curves around the medial condyle of the tibia where it becomes flattened, and inserts into the upper part of the medial surface of the body of the tibia, below the condyle (Fig. 30.1). As a result, the muscle is mainly a lower limb adductor. However, since adduction depends on several thigh muscles, limb function can be adequately preserved after harvest of the gracilis muscle.
The gracilis is supplied by a branch of the medial circumflex artery (which comes from the profunda femoral system), and its innervation comes from the anterior branch of obturator nerve, originating at L3 and L4 spinal roots. Both the main arterial supply and the innervation enter the muscle at the same site, or approximately 1 cm apart, at a region termed the neurovascular bundle (Fig. 30.2). This bundle enters the muscle in its proximal third, on average approximately 10 cm from its pubic origin. The proximal location of the neurovascular bundle, situated adjacent to the groin, combined with the fact that thigh motion can be preserved after harvesting, makes this long muscle an ideal candidate for a rotational flap transposition to the perineal region. In most patients, accessory small perforating vessels may enter the muscle distal to the neurovascular
bundle (4). However, division of these vessels is possible in more than 90% of cases, thus preserving muscle viability.
bundle (4). However, division of these vessels is possible in more than 90% of cases, thus preserving muscle viability.
 Figure 30.1 Anatomy of the gracilis muscle. A. A sketch showing the bony structures and the gracilis. B. A sketch showing the bony structures, adjacent muscles, and the gracilis. |
Alternative Options for the Repair of Rectourethral and Rectovaginal Fistulas
These techniques may be divided into two main categories. In the first, the luminal side of the fistula is approached, and the defect is closed. Rectovaginal fistulas may be approached to either repair the rectal side of the fistula, or through the vagina, to repair the vaginal opening (5). The advantage of the vaginal approach is the ease of access to the fistula opening. However, this approach carries the disadvantage of repairing the lower pressure side of the fistula. For this reason, most colorectal surgeons prefer approaches involving repair of the rectal side. In the rectourethral fistula, the urethral side is not accessible, and direct repair of the fistula opening must involve repair of the rectal side. The rectal lumen may be approached through the anus, or by incision of the posterior wall of the rectum. This posterior method entailed either a transsphincteric plane, or a transsacral incision. Several authors have advocated the posterior York-Mason approach to the rectum (6). The major drawback of the posterior and transanal approaches is that they mainly treat the rectal side of the fistula. Unfortunately, the high-pressure side in rectourethral fistula is the urethra.
In the second category of repair, the plane between the rectum and the urethra is dissected, the fistula is divided, and both the rectal and the urethral defects are repaired. A viable tissue flap may then be transposed to separate the rectum and the urethra. The greater omentum may be used as a viable flap (7), but this use involves a laparotomy, with deep anterior pelvic dissection, and may not be feasible in patients who have had abdominal surgeries. Using the perineal approach, the plane between the rectum and the vagina or urethra is dissected through a perineal incision, the fistula is divided, and a viable tissue is brought to interpose between the two organs. The gracilis muscle provides a well-vascularized muscular rotation flap and avoids the need for laparotomy.
A relatively new option for interposition between the rectum and the urethra or the vagina without body tissue flaps may involve the use of biologic meshes. These meshes are acellular collagen matrixes made of biologic tissue, which may be used as “tissue grafts” (8). Wound-healing processes result in migration of inflammatory cells and fibroblasts into the mesh, leading to dense fibrosis with gradual absorption of the mesh. However, since this technique has only recently emerged, data are still insufficient to support its routine use. Biologic collagen products such as the collagen plugs, also made from acellular collagen matrixes, may also be used for the repair of rectovaginal fistulas by simple insertion of the plug throughout the fistula tract (9). The short length of rectovaginal fistulas may make fixation of the plug to the fistula tract difficult and increase the chance of plug migration. Newer products use a fixation button attached to the plug, aiming to reduce the chance of such migration.
Assessment of Patients with Rectovaginal Fistula
Patients with fistula between the rectum and the vagina often complain of uncontrolled passage of gas and stool from the vagina. Large fistulas may be easily evident on physical examination, using digital rectal examination, anoscopy, or examination of the vagina using speculum. Small fistulas however may be clinically difficult to detect. In these cases, imaging examinations may be required to definitely identify the fistula tract. A water-soluble contrast enema of the rectum, or vaginogram, using a catheter inserted into the lower part of the vagina and instillation of water-soluble contrast into the vagina, may show the fistula tract. Transrectal or perineal sonography and pelvic magnetic resonance imaging (MRI) are modern imaging techniques with high sensitivity to detect the fistula tract. However, such modern imaging techniques may not be available to all surgeons and are more costly. Infrequently, the fistula tract cannot be detected by physical examination or by imaging techniques, and examination under anesthesia is required to detect the fistula tract. In such examinations, probing of the fistula tract may be attempted through the anal or the vaginal side. If the openings are not identified, insufflation of the rectum or the vagina with Betadine or colored solution may be used, in attempts to detect passage of the colored solution at the other opening. Alternatively, a tampon soaked with colored solution may be inserted to the vagina approximately 1 hour prior to the examination, to enhance identification of the rectal opening.
Once diagnosed, adequate drainage of the rectovaginal or pouch-vaginal fistula must be assured, prior to any attempt of repair. If physical examination or imaging procedures suggest inadequately drained fistula, or associated cavity, adequate drainage must first be achieved, by incision and drainage, insertion of draining Seton, or both. In patients with inadequate drainage who are not diverted, temporary fecal diversion may be considered, to improve local conditions prior to fistula repair.
Patients with rectovaginal fistula associated with Crohn’s disease may have active proctitis or aggressive perianal disease, with unfavorable local conditions negatively affecting success rate of fistula repair. In these cases, adequate anti-inflammatory treatment for Crohn’s disease should be promptly initiated prior to the fistula repair.
Assessment of Patients with Rectourethral Fistula
Rectourethral fistulas often present with symptoms such as pneumaturia, fecaluria, passage of urine through the rectum, and recurrent urinary tract infections. Although most of these symptoms are often alleviated by fecal and urinary diversion, the fistulas seldom spontaneously heal. Even when diverted, patients may suffer from urinary tract infections, resistant to medical therapy (10). Thus, most of these patients will eventually require surgical repair. Similar to patients with rectovaginal fistulas, the rectal opening of fistulas between the rectum and the urethra may or may not be evident on clinical examination. Imaging studies, including water-soluble contrast enema, voiding cystourethrography, transrectal ultrasonography, or pelvic MRI may be required for identification of fistula. Infrequently, examination under anesthesia with the combination of cystourethroscopy and anorectal examination may be required. In these cases, fluid installed into the urethra may help identify the rectal opening.
In patients with a history of malignant disease, such as patients with rectourethral fistula following treatment for prostate cancer, or patients with pouch-vaginal fistula following surgery for rectal cancer, recurrent malignancy causing the fistula must be excluded. Preoperative biopsies of the fistula tract, imaging modalities, and serum tumor markers may be appropriate to rule out recurrent cancer.
Assessment of Patients with Nonhealing Perineal Wounds
As discussed earlier in the chapter, the first step in the preoperative evaluation of patient with a history of rectal cancer is to exclude recurrence. Subsequently, the depth of the wound should be evaluated. A well-mobilized gracilis will reach up to 20 cm cephalad to the gluteal cleft, any wound deeper than that should be treated with a different approach. The best method is probably a rectus abdominis myocutaneous flap. Computed tomographic scan should also be obtained to exclude the presence of undrained abscess cavities. The addition of oral and intravenous contrast may be helpful if an enteric fistula is suspected. MRI and fistulogram can be used to further evaluate other associated defects.
Preoperative Preparations
Numerous surgical procedures have been described for the treatment of rectourethral and rectovaginal fistulas (6,11,12), none of which has gained wide acceptance as the procedure of choice. Thus, each treatment option has its own associated advantages and risks, all of which should be thoroughly discussed with the patient before obtaining informed consent.
The value of fecal diversion prior to or concomitant with the fistula repair has not been adequately challenged in comparative studies (13). The rationale behind fecal diversion is that decreased fecal load may reduce the chance of postoperative infection, and will also prevent rise of luminal pressure at the rectal side during evacuation. Most authors believe that fecal diversion is recommended, since the perineal approach includes extensive dissection of the tissue between the rectum and the vagina or the urethra. The importation of healthy muscle has a high chance of success, which may be further enhanced by routine fecal diversion. Until comparative trials are available, the authors and the editors advocate routine fecal diversion.
In rectourethral fistulas, urinary diversion using cystostomy should also be considered, to prevent any voiding attempts that may raise the luminal pressure at the urethral side.
Broad-spectrum intravenous antibiotic prophylaxis is given prior to the induction of anesthesia. The length of postoperative prophylactic antibiotic treatment, if any, is not scientifically well defined, and there is no proof that prolonged antibiotic treatment improves healing rate, although antibiotic treatment for 24 hours or more is often used.
The same antibiotic coverage is used when operations are performed for persistent sinuses. Patients with persistent perineal sinus are typically colonized with skin contaminants, such as Staphylococcus aureus, diphteroids, β-streptococcus, and Staphylococcus epidermidis. Anaerobic Bacteroides are present in 25% of the wounds and anaerobic gram-negative rods are rarely seen (14).
If a patient has not been diverted prior to fistula repair, mechanical bowel preparation may be advisable, for large bowel cleansing prior to surgery. Alternatively, phosphate enemas may be used to clean the rectum and sigmoid colon.
Surgery
Harvesting of the Gracilis Rotation Flap
Harvesting for Fistula Repair
When used for fistula repair, the gracilis muscular flap is used, without any overlaying skin. The patient is positioned either in the supine position with the legs adducted, or in the modified lithotomy position using stirrups. Several types of thigh incisions may
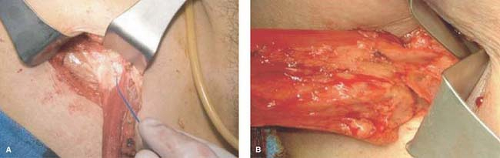
be used for the harvest procedure. In relatively thin patients, where the muscle is easily palpated, two to three 3–5 cm long incisions can be made alongside the inner part of the thigh over the gracilis muscle (Fig. 30.3). Tunnels are made in-between the incisions at the subcutaneous tissue overlaying the muscle, and the muscle is dissected throughout its length through these incisions (Fig. 30.4). The perforating vessels are divided through these small incisions often with an energy source. Using an energy sources, perforating vessels entering the muscle between the incision sites can safely be divided through small incisions. The most proximal incision is situated approximately one hand breadth beneath the inguinal ligament, to allow adequate exposure of the neurovascular bundle. Alternatively, a longer incision of approximately 10–12 cm may be made at the upper part of the inner thigh, to enhance exposure and facilitate dissection at the area of the neurovascular bundle. In this case, a second incision is made at the distal part of the thigh, and a subcutaneous tunnel is made in between these two incisions. In difficult cases, or if the surgeon does not feel confident dissecting parts of the muscle through a subcutaneous tunnel, a long incision may be made along the inner part of the thigh.
be used for the harvest procedure. In relatively thin patients, where the muscle is easily palpated, two to three 3–5 cm long incisions can be made alongside the inner part of the thigh over the gracilis muscle (Fig. 30.3). Tunnels are made in-between the incisions at the subcutaneous tissue overlaying the muscle, and the muscle is dissected throughout its length through these incisions (Fig. 30.4). The perforating vessels are divided through these small incisions often with an energy source. Using an energy sources, perforating vessels entering the muscle between the incision sites can safely be divided through small incisions. The most proximal incision is situated approximately one hand breadth beneath the inguinal ligament, to allow adequate exposure of the neurovascular bundle. Alternatively, a longer incision of approximately 10–12 cm may be made at the upper part of the inner thigh, to enhance exposure and facilitate dissection at the area of the neurovascular bundle. In this case, a second incision is made at the distal part of the thigh, and a subcutaneous tunnel is made in between these two incisions. In difficult cases, or if the surgeon does not feel confident dissecting parts of the muscle through a subcutaneous tunnel, a long incision may be made along the inner part of the thigh.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree