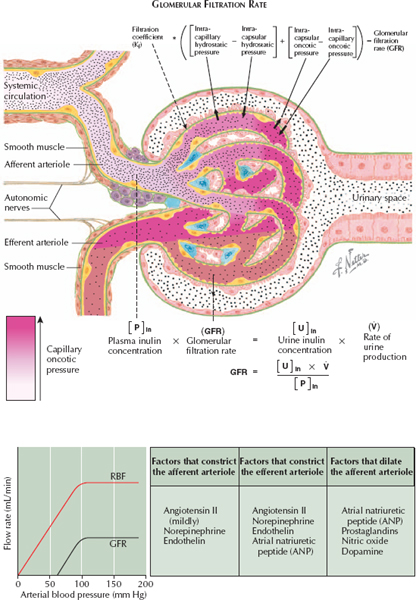
GLOMERULAR FORCES THAT DETERMINE FILTRATION RATE
Blood that enters the glomerular capillaries is filtered into Bowman’s space, the first region of the nephron. The glomerular filtration rate (GFR) is equal to the volume of plasma filtered through the capillary walls per unit of time, and it depends on the pressure differential (both hydrostatic and oncotic) between the glomerular capillaries and Bowman’s space. Hydrostatic pressure in the glomerular capillaries, as well as oncotic pressure in Bowman’s space, favor filtration. In contrast, hydrostatic pressure in Bowman’s space, as well as oncotic pressure in the glomerular capillaries, oppose filtration.
These relationships can be expressed using the following equation:
GFR = Kf [(Pgc − Pbs) + (πbs − πgc)]
Kf = filtration coefficient
P = hydrostatic pressure
p = oncotic pressure
gc = glomerular capillaries
bs = Bowman space
The filtration coefficient Kf depends on characteristics of the glomerular capillary walls, such as total surface area and permeability. Thus processes that promote loss of glomeruli, such as glomerulosclerosis, or thickening of the capillary walls, such as diabetes mellitus, lower the GFR.
Pgc depends on systemic arterial pressure and the resistance in the afferent and efferent arterioles. It is constant along the length of the glomerular capillaries.
πgc depends on plasma oncotic pressure, and it increases as fluid approaches the efferent arteriole because removal of plasma fluid concentrates nonfilterable proteins. As pgc increases, the forces favoring and opposing filtration eventually become equal. This point is known as filtration equilibrium.
Both Pgc and πgc change in response to constriction or dilation of the afferent and efferent arterioles. For example, constriction of the efferent arteriole increases Pgc but reduces renal plasma flow, which slows the rate at which blood passes through the glomerular capillaries. Because plasma therefore spends more time in contact with the filtration surface, πgc rises more rapidly along the length of the capillaries. Thus the GFR remains largely constant because the changing hydrostatic and oncotic forces are balanced. In contrast, constriction of the afferent arteriole decreases Pgc and causes an earlier rise in πgc, decreasing the GFR. Finally, dilation of the afferent arteriole increases Pgc and causes a later rise in πgc, increasing the GFR.
Several agents alter tone in the afferent vessels. The most important include angiotensin II (AII), which preferentially constricts the efferent arteriole, maintaining the GFR constant; norepinephrine (i.e., sympathetic input), which constricts both afferent and efferent arterioles, reducing the GFR; atrial natriuretic peptide (ANP), which dilates the afferent arteriole and constricts the efferent arteriole, increasing the GFR; and prostaglandins, which dilate the afferent arteriole, increasing the GFR.
Under normal circumstances, the pressures in Bowman’s space are less variable and less important than those in the glomerular capillaries. Pbs depends on pressures throughout the urinary collecting system and may be increased in the presence of a urine outflow obstruction. Normally, πbs is negligible.