General Features of the Gastrointestinal Tract and Evaluation of Specimens Derived from It
General Comments
In many ways the gastrointestinal (GI) tract is a remarkable organ. The embryonic endoderm will give rise to the future GI tract. The GI tract will acquire multiple cell types that will be dispersed into two planes: A vertical plane that allows one to recognize different layers of the bowel wall and a horizontal plane that develops into the esophagus, stomach, small intestine, colon, and anus. Although these cell types will resemble one another, important histologic differences allow specific physiologic functions to be carried out in each anatomic region. Interactions between cell populations regulate subsequent patterns of gene expression and organ development (1).
The physiology of the GI tract is also impressive. It serves as the digestive organ of the body, taking in everything that is swallowed and turning it into useful nutrients or discarding what is left over as waste. These processes begin in the mouth and terminate at the anus. While digesting everything to which it is exposed and breaking it down into smaller, absorbable, chemical substances, the gut is itself able to withstand these processes and avoid autodigestion. Complex neuromuscular interactions allow the GI tract to move food and liquids from one section of the gut to the next while at the same time controlling the passage of food in such a way that maximum digestion and absorption occurs in each of the appropriate spots. Even in a single organ, such as the small intestine, a differentiation gradient exists such that different substances are preferentially absorbed at different intestinal sites and through different parts of the cell. Not everything taken into the mouth and swallowed is healthy for the patient. Therefore, the GI tract serves as a major interface between the outside world and the rest of the body. The gut is continuously exposed to toxins and infectious organisms, yet it is often capable of eliminating these agents without causing any harm to itself. Not surprisingly, breakdown in these defense processes often results in disease. This generally occurs when the integrity of the bowel wall becomes compromised. The gut also serves as a major immune organ. It is the major site of the generation of mucosal immunity, hence the utility of oral vaccines. These immunologic processes largely take place in the small bowel. Finally, the GI tract is a major endocrine organ.
In humans, the gut is divided into four major organs: Esophagus, stomach, small intestine, and large intestine. These are separated by sphincters that control the passage of contents from one organ to the next. The junctions between organs are identifiable by an abrupt change in the mucosal nature and by the presence of the sphincters.
Embryology
Multiple interactions occur during development between and within the three germ layers (endoderm, mesoderm, and ectoderm). Each of these layers may reciprocally induce development of other layers. The endoderm induces the mesoderm (2). It also confers a dorsal-ventral pattern on it (2). The endoderm and ectoderm contact one another in the 2- to 4-week embryo. The endoderm, which forms the roof of the yolk sac, gives rise to the future gut, creating the majority of the epithelial lining of the GI tract, biliary passages, liver, and pancreas. The primitive gut temporarily consists of foregut, midgut, and hindgut. The splanchnic mesoderm surrounding the primitive gut forms the muscular and connective tissue layers. Embryologic development takes place with a large number of inducers that share overlapping expression patterns and redundant functions. The core of this system consists of a set of structurally similar genes known as homeobox genes. The embryology of each region is discussed in detail in the relevant chapters.
Multipotential neural progenitors give rise to various derivatives, including neurons, glia, and ganglia, as the result of local environmental signals. These progenitors differentiate based on specific molecules they encounter either during their migration or within the organ in which they terminally differentiate as discussed in detail in Chapter 10.
Abundant endocrine cells are present by 8 weeks’ gestation. The diversity of the GI endocrine cell component is established early. There is a higher density of endocrine cells in both the proximal duodenum and distal colon/rectum compared with other areas. Endocrine cell numbers increase with age, roughly paralleling the growth of the gut. The full adult profile is attained by the second trimester. The endocrine system is discussed in detail in Chapter 17.
Peripheral lymph nodes develop by the second month of gestation. Mononuclear cell aggregates are also identified in the fetal intestine in proximity to the endodermal epithelium.
Cell Proliferation
The GI tract undergoes continuous development and proliferation. Its mucosa varies from the esophagus to the colon and it contains multiple cell types. New cells form from stem cells within the basal layers of the anal and esophageal squamous epithelium, in the mucous neck region of the stomach, or from the bases of the crypts in the intestines (Fig. 1.1). Cells then migrate out of the proliferative zone and differentiate into multiple cell lineages. The differentiation pattern of the gut epithelium is influenced by other cells in its environment (3). Eventually the cells are shed into the lumen or undergo apoptosis. Cellular life span is 5 to 7 days in the duodenum and jejunum, 4 to 5 days in the ileum, and 4 to 6 days in the large intestine. Specific details of cell proliferation in the esophagus, stomach, small intestine, and colon are discussed in the relevant chapters.
The intestine alters its rates of cellular renewal and adapts to surgical, nutritional, and other toxic stimuli, as well as to physiologic and disease states. Stem cells show three general features: (a) the capacity to undergo asymmetric division, producing one daughter that remains a stem cell and another that undergoes a seemingly irreversible commitment to enter a differentiation pathway; (b) proliferative potential; and (c) the ability to retain a position in a particular environmental niche (4,5). Some stem cells are unipotential, giving rise to a single differentiated phenotype, whereas others are multipotential, giving rise to multiple cell types.
Of interest is the fact that not all stem cells that give rise to gastrointestinal epithelia are gastrointestinal stem cells. It now appears that bone marrow stem cells may be recruited to sites of injury and give rise to a host of cell lineages, including cells with mixed gastrointestinal phenotypes. This phenomenon is best exemplified in the development of gastric cancer following Helicobacter pylori infections as discussed in Chapter 5.
Gastrointestinal Structure
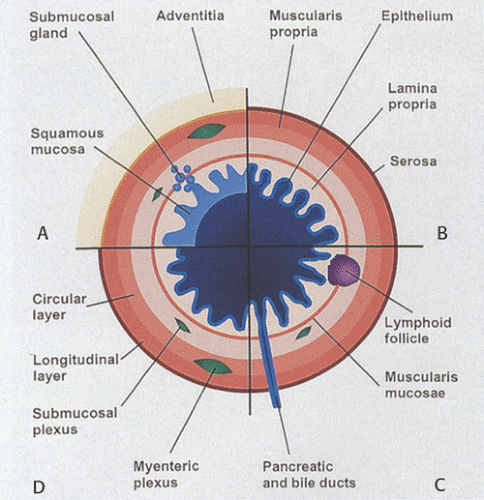
In general, the gut consists of four concentric layers as one progresses outward from the lumen: The mucosa, submucosa, muscularis propria, and serosa or adventitia (Fig. 1.2). These layers are easily visualized histologically and can also be imaged using ultrasound. The high correlation that exists between the ultrasound image and the histologic features allows one to use ultrasound to provide diagnostic information about the status of the GI tract.
Mucosa
The mucosa consists of an epithelial lining, a lamina propria that contains loose connective tissue rich in immunocompetent cells, and the muscularis mucosae. The lamina propria is most visible in the stomach, large and small intestines, and appendix, and is least visible in the esophagus and anus. The smooth muscle cells in the muscularis mucosae are predominantly arranged in a circular orientation, although some longitudinal muscle fibers are also present.
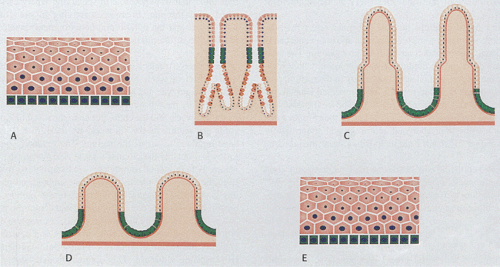
The epithelium may invaginate to form glands that extend into the (a) lamina propria (i.e., mucosal glands in the stomach) (Fig. 1.2), (b) submucosa (i.e., submucosal glands in the esophagus [Fig. 1.2] or Brunner glands in the duodenum), or (c) ducts that extend to organs outside the gut, such as to the pancreas or the liver. The mucosa and submucosa may also project into the GI lumen as folds (plicae or rugae) (Fig. 1.2). Additionally, villi may be present (Fig. 1.2).
GI epithelium differs substantially in various organs. Squamous epithelium lines the mucosa of the esophagus and anus (Fig. 1.3). In the stomach, the epithelium is divided into surface epithelium, pits, and glands (Fig. 1.3). In the small intestine, the mucosa consists of crypts and villi containing a population of intestinal epithelial cells. The colon contains similar cells but it lacks villi. The squamous lining of the esophagus protects it from the passage of undigested food over its surface. Likewise, in the anus, the squamous epithelium protects the mucosa from the damaging effects of the passage of solid waste. In the stomach, the mucosa facilitates digestion by secreting acid. The epithelial lining of the small intestine is uniquely suited to the further digestion and absorption of nutrients along a gradient from the duodenum to the ileum. The colon predominantly resorbs water. The specific features of the various portions of the gut are discussed in their appropriate chapters.
The lamina propria forms the mucosal interglandular tissues. It appears as a delicate, loose, connective tissue containing lymphocytes, plasma cells (Fig. 1.4), eosinophils, rare neutrophils, and mast cells. The majority of the cells are plasma cells and lymphocytes, with the majority of plasma cells secreting IgA; however, IgM-, IgG-, and IgE-secreting cells are also present. The lamina propria also contains large numbers of macrophages, which play important roles in mucosal immunity, antigen presentation, elimination of exogenous pathologic antigens or organisms, immunoglobulin production, and immunoregulation (6).
The gut-associated lymphoid tissue (GALT) primarily lies within the lamina propria. It is distributed diffusely or appears as solitary (Fig. 1.5) or aggregated nodules, which in the ileum and appendix are called Peyer patches. Larger aggregates contain germinal centers. Peyer patches are present on the antimesenteric border of the intestine. These lymphoid nodules often span the muscularis mucosae (Fig. 1.5), breaking through into the superficial submucosa and creating gaps in the muscularis mucosae. Solitary nodules occur in the esophagus, in the gastric pylorus, and along the small and large intestines. Both blood and lymphatic capillaries form a plexus around the lymphoid follicles (7). The majority of the cells within the follicles are lymphocytes, macrophages, and plasma cells. The basement membrane overlying the Peyer patches is more porous than that of the adjacent epithelial areas, facilitating the bidirectional passage of lymphocytes during antigenic stimulation (8). The lamina propria also contains vessels and unmyelinated nerve fibers.
Mast cells comprise an important, but heterogeneous, component of the lamina propria and submucosa. Specialized contacts exist between mast cells and other cell types; these contacts facilitate intercellular communication. Mast cells often lie adjacent to blood vessels or lymphatics, near or within nerves, and beneath epithelial surfaces, particularly those of the GI tract where they are exposed to environmental antigens (9,10). Numerous cells respond to mast cell mediators, as exemplified by the presence of histamine receptors on their surfaces. Cells with surface histamine receptors include lymphocytes, macrophages, neutrophils, basophils, eosinophils, smooth muscle cells, and parietal cells. Neuropeptides and hormones influence mast cells, inducing them to release their mediators.
Mast cells initiate acute inflammation and propagate chronic inflammatory changes. These cells may also be antigen-presenting cells. In some IgE-dependent inflammatory responses, mast cells are the sole or primary initiator of the reaction, and in others they may influence pre-existing inflammation. By virtue of their location and number, mast cells participate in a wide variety of GI diseases. The most important include food allergies, eosinophilia, immunodeficiency
syndromes, immediate hypersensitivity reactions, host responses to parasites and neoplasms, immunologically nonspecific inflammatory and fibrotic conditions, and tissue remodeling. Mast cells are also important in angiogenesis, wound healing, peptic ulcer disease, and other chronic inflammatory conditions, including graft versus host disease and inflammatory bowel disease.
syndromes, immediate hypersensitivity reactions, host responses to parasites and neoplasms, immunologically nonspecific inflammatory and fibrotic conditions, and tissue remodeling. Mast cells are also important in angiogenesis, wound healing, peptic ulcer disease, and other chronic inflammatory conditions, including graft versus host disease and inflammatory bowel disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree