CHAPTER 1 Gastrointestinal Hormones and Neurotransmitters
CELLULAR COMMUNICATION
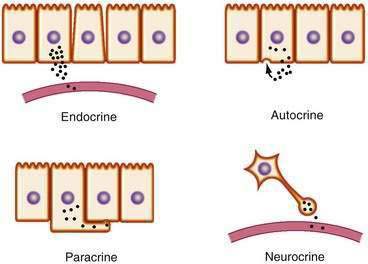
Chemical transmitters of the gut are produced by discrete cells of the GI mucosa and can be classified as endocrine, paracrine, synaptic (“neurocrine”), or autocrine (Fig. 1-1). Specialized signaling cells that secrete transmitters into the blood are known as endocrine cells, and the transmitters they produce are known as hormones. Hormones bind to specific receptors on the surface of target cells at remote sites and regulate metabolic processes.1
In contrast with endocrine cells that act on distant target tissues, other signaling cells of the GI tract may produce transmitters that act on neighboring cells. This process is known as paracrine signaling and is typical of cells that produce somatostatin.2 Paracrine transmitters are secreted locally and cannot diffuse far. They bind to receptors on nearby cells to exert their biological actions. These actions are limited because they are taken up rapidly by their target cells, destroyed by extracellular enzymes, and adhere to extracellular matrix, all of which limit their ability to act at distant sites. Because paracrine signals act locally, their onset of action is generally rapid and can be terminated abruptly. By comparison, endocrine signaling takes much longer, and termination of signaling requires clearance of hormone from the circulation.
Endocrine transmitters of the GI tract consist predominantly of peptides (e.g., gastrin, secretin). Paracrine transmitters can be peptides, such as somatostatin, or nonpeptides, such as histamine, that act locally on neighboring cells. Neurotransmitters can be peptides, such as vasoactive intestinal polypeptide (VIP) and tachykinins, or small molecules, such as acetylcholine and norepinephrine, that are secreted, or nitric oxide (NO), which simply diffuses across the synaptic cleft. The major transmitters and hormones of the GI tract are listed in Table 1-1.
Table 1-1 Hormones and Transmitters of the Gastrointestinal Tract
Peptides That May Function as Hormones, Neuropeptides, or Paracrine Agents
Some cells release messengers locally and possess cell surface receptors for the same messengers, thus enabling those cells to respond to their own secreted products. This mode of transmission, known as autocrine signaling, has been demonstrated for several growth factors and has been implicated in the growth of certain cancers, including colorectal cancer (see Chapter 3).3
NEURAL REGULATION OF THE GI TRACT
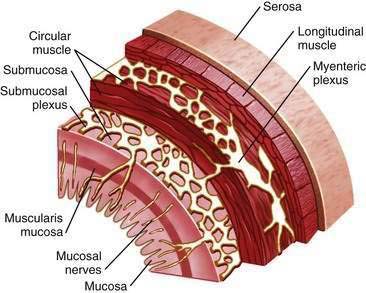
The enteric nervous system plays an integral role in the regulation of gut mucosal and motor function.4 It is organized into two major plexuses (Fig. 1-2). The myenteric plexus lies between the external longitudinal and internal circular muscle layers. The submucosal plexus lies between the circular muscle layer and the mucosa. Although the enteric nervous system receives input from the central and autonomic nervous systems, it can function independently. Nerves of the myenteric plexus project fibers primarily to the smooth muscle of the gut, with only a few axons extending to the submucosal plexus. Most of the fibers of the submucosal plexus project into the mucosa and the submucosal and myenteric plexuses. Various peptide and nonpeptide neurotransmitters are found in the enteric nervous system. Studies using immunohistochemical staining have localized neurotransmitters to specific neurons in the GI tract. γ-Aminobutyric acid is found primarily in the myenteric plexus and is involved in regulating smooth muscle contraction. Serotonin is found within the plexus and functions as an interneuron transmitter. Adrenergic neurons originate in ganglia of the autonomic nervous system and synapse with enteric neurons. Peptides such as neuropeptide Y (NPY) are often secreted from the same adrenergic neurons and generally exert inhibitory effects, such as vasoconstriction.5 Other adrenergic neurons containing somatostatin project to the submucosal plexus, where they inhibit intestinal secretion. Coexistence of peptides and neurotransmitters in the same neurons is not unusual; in fact, the interplay among transmitters is critical for coordinated neural regulation.6 For example, the peptides VIP and peptide histidine isoleucine (PHI) are commonly found together, as are the tachykinins substance P and substance K, where they have complementary effects.
The ability of hormones to act on nerves locally within the submucosa of the intestine and affect more distant sites on nerves such as the vagus expands the potential organs that may be regulated by gut hormones.7 Chemical and mechanical stimuli cause the release of hormones from endocrine cells of the intestinal mucosa. These interactions initiate a wide variety of secretomotor responses, many of which are mediated by enteric neurons. Secretomotor circuits consist of intrinsic primary afferent neurons with nerve endings in the mucosa and extension through the myenteric and submucosal plexi. This circuitry allows nerves to stimulate mucosal cells to secrete fluid and electrolytes and at the same time stimulate muscle contraction. The same motor neurons also have axons that supply arterioles and can initiate vasodilator reflexes.
Extrinsic primary afferent neurons can be of the vagus, with somal bodies in the nodose ganglia and axons that reach the gut through the vagus nerve, or of the spinal nerves of the thoracic and lumbar regions, whose cell bodies lie in the dorsal root ganglia. Information conducted by extrinsic primary afferent neurons includes pain, heat, and sensations of fullness or emptiness. These neurons are also targets for hormones. For example, the satiety effect of CCK in the bloodstream is mediated through the vagus nerve.8 Specific CCK receptors have been identified on the vagus, and blockade of these receptors abolishes the satiation induced by peripheral CCK.
Endocrine, paracrine, and neural transmitters existing within the lamina propria modulate effects on the gut immune system.7 Lymphocytes, macrophages, mast cells, neutrophils, and eosinophils are potential targets for endocrine and neural transmitters and participate in the inflammatory cascade. Moreover, inflammatory mediators can act directly on enteric nerves. Serotonin released from endocrine cells is involved in intestinal anaphylaxis and stimulates vagal afferent fibers that possess the 5-hydroxytryptamine 3 (5-HT3) receptor.
PEPTIDE HORMONES OF THE GI TRACT
SYNTHESIS, POST-TRANSLATIONAL MODIFICATION, AND SECRETION
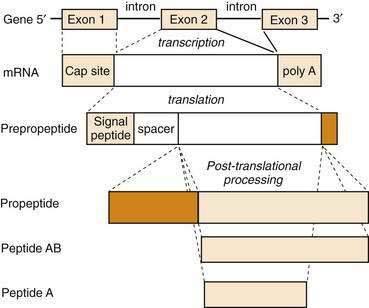
All GI peptides are synthesized via gene transcription of DNA into messenger RNA (mRNA) and subsequent translation of mRNA into precursor proteins known as preprohormones. Peptides that are to be secreted contain a signal sequence that directs the newly translated protein to the endoplasmic reticulum, where the signal sequence is cleaved and the prepropeptide product is prepared for structural modifications.9 These precursors undergo intracellular processing and are transported to the Golgi apparatus and packaged in secretory granules. Further modifications in peptide structure may occur within the Golgi apparatus (e.g., sulfation) that is important for the bioactivity of many peptide hormones, such as CCK. Secretory granules may be targeted for immediate release or stored in close proximity to the plasma membrane for release following appropriate cell stimulation. When GI endocrine cells are stimulated, mature hormone is secreted into the paracellular space and is taken up into the bloodstream. For many hormones, such as gastrin and CCK, multiple molecular forms exist in blood and tissues. Although there is only a single gene for these peptides, the different molecular forms result from differences in pretranslational or post-translational processing (Fig. 1-3). A common mechanism of pretranslational processing includes alternative splicing of mRNA, which generates unique peptides from the same gene. Post-translational changes include cleavage of precursor molecules.
Enzymatic cleavage of the signal peptide produces a prohormone. Other post-translational features that result in mature GI peptides include peptide cleavage to smaller forms (e.g., somatostatin), amidation of the carboxyl terminus (e.g., gastrin), and sulfation of tyrosine residues (e.g., CCK). These processing steps are usually critical for biological activity of the hormone. For example, sulfated CCK is 100-fold more potent than its unsulfated form. The vast biochemical complexity of gastroenteropancreatic hormones is evident in the different tissues that secrete these peptides. As GI peptides are secreted from endocrine as well as nervous tissue, the distinct tissue involved often determines the processing steps for production of the peptide. Many hormone genes are capable of manufacturing alternatively spliced mRNAs or proteins that undergo different post-translational processing and ultimately produce hormones of different sizes. These modifications are important for receptor binding, signal transduction, and consequent cellular responses.10
It has become possible to express human genes in other species. By introducing specific hormone-producing genes into pigs or sheep, human hormones have been produced for medicinal use.11 With the rapid sequencing of the human genome, it is likely that novel methods of gene expression will expand the therapeutic use of human proteins. Moreover, drugs are being developed that inhibit the transcription of DNA into mRNA or that block the gene elements responsible for turning on specific hormone production (e.g., antisense oligonucleotides).12 This technology is based on the principle that nucleotide sequences bind to critical DNA regions and prevent transcription into mRNA. Similarly, oligonucleotides can be made to interact with mRNA and alter (or inhibit) translation of a protein product. These principles may be applicable to the treatment of the growing list of diseases that result from aberrant protein processing.13,14
GASTRIN
As discussed in more detail in Chapter 49, gastrin is the major hormone that stimulates gastric acid secretion. Subsequently, gastrin was found to have growth-promoting effects on the gastric mucosa and possibly some cancers.15 Human gastrin is the product of a single gene located on chromosome 17. The active hormone is generated from a precursor peptide called preprogastrin. Human preprogastrin contains 101 amino acids (AAs), including a signal peptide (21 AAs), spacer sequence (37 AAs), gastrin component (34 AAs), and a 9-AA extension at the carboxyl terminus. The enzymatic processing of preprogastrin produces all the known physiologically active forms of gastrin.
Preprogastrin is processed into progastrin and gastrin peptide fragments of various sizes by sequential enzymatic cleavage. The two major forms of gastrin are G34 and G17, although smaller forms exist. The common feature of all gastrins is an amidated tetrapeptide (Try-Met-Asp-Phe-NH2) carboxyl terminus, which imparts full biological activity. Modification by sulfation at tyrosine residues produces alternative gastrin forms of equal biological potency. A nonamidated form of gastrin known as glycine-extended gastrin is produced by colonic mucosa. Glycine-extended gastrin has been shown in animal models to stimulate proliferation of normal colonic mucosa and enhance the development of colorectal cancer. It is not known whether local production of this form of gastrin contributes to human colon carcinogenesis, and the receptor for glycine-extended gastrin has not been identified.16
Most gastrin is produced in endocrine cells of the gastric antrum.17 Much smaller amounts of gastrin are produced in other regions of the GI tract, including the proximal stomach, duodenum, jejunum, ileum, and pancreas. Gastrin has also been found outside the GI tract, including in the brain, adrenal gland, respiratory tract, and reproductive organs, although its biological role in these sites is unknown.
The receptors for gastrin and CCK are related and constitute the so-called gastrin-CCK receptor family. The CCK-1 and CCK-2 (previously known as CCK-A and -B) receptor complementary DNAs were cloned from the pancreas and brain, respectively, after which it was recognized that the CCK-2 receptor is identical to the gastrin receptor of the stomach.18
Hypergastrinemia occurs in pathologic states associated with decreased acid production, such as atrophic gastritis. Serum gastrin levels can also become elevated in patients on prolonged acid-suppressive medications, such as histamine receptor antagonists and proton pump inhibitors. Hypergastrinemia in these conditions is caused by stimulation of gastrin production by the alkaline pH environment. Another important but far less common cause of hypergastrinemia is a gastrin-producing tumor, also known as Zollinger-Ellison syndrome (see Chapter 32).
The gastrin analog, pentagastrin, has been used clinically to stimulate histamine and gastric acid secretion in diagnostic tests of acid secretory capacity (see Chapter 49).
CHOLECYSTOKININ
CCK is a peptide transmitter produced by I cells of the small intestine and is secreted into the blood following ingestion of a meal. Circulating CCK binds to specific CCK-1 receptors on the gallbladder, pancreas, smooth muscle of the stomach, and peripheral nerves to stimulate gallbladder contraction and pancreatic secretion, regulate gastric emptying and bowel motility, and induce satiety.19 These effects serve to coordinate the ingestion, digestion, and absorption of dietary nutrients. Ingested fat and protein are the major food components that stimulate CCK release.
CCK was originally identified as a 33–amino acid peptide. However, since its discovery larger and smaller forms of CCK have been isolated from blood, intestine, and brain. All forms of CCK are produced from a single gene by post-translational processing of a preprohormone. Forms of CCK ranging in size from CCK-58 to CCK-8 have similar biological activities.20
CCK is the major hormonal regulator of gallbladder contraction. It also plays an important role in regulating meal-stimulated pancreatic secretion (see Chapter 56) In many species, this latter effect is mediated directly through receptors on pancreatic acinar cells but in humans, in whom pancreatic CCK-1 receptors are less abundant, CCK appears to stimulate pancreatic secretion indirectly through enteropancreatic neurons that possess CCK-1 receptors. In some species, CCK has trophic effects on the pancreas, although its potential role in human pancreatic neoplasia is speculative. CCK also has been shown to delay gastric emptying.21 This action may be important in coordinating the delivery of food from the stomach to the intestine. CCK has been proposed as a major mediator of satiety and food intake, an effect that is particularly noticeable when food is in the stomach or intestine. CCK inhibits gastric acid secretion by binding to CCK-1 receptors on somatostatin (D) cells in the antrum and oxyntic mucosa. Somatostatin acts locally to inhibit gastrin release from adjacent G cells and directly inhibits acid secretion from parietal cells.22
Clinically, CCK has been used together with secretin to stimulate pancreatic secretion for pancreatic function testing. It is also used radiographically or scintigraphically to evaluate gallbladder contractility. There are no known diseases of CCK excess. Low CCK levels have been reported in individuals with celiac disease who have reduced intestinal mucosal surface area and in those with bulimia nervosa.23,24 Elevated levels of CCK have been reported in some patients with chronic pancreatitis (see Chapter 59), presumably because of reduced pancreatic enzyme secretion and interruption of negative feedback regulation of CCK release.25
SECRETIN
The first hormone, secretin, was discovered when it was observed that intestinal extracts, when injected intravenously into dogs, caused pancreatic secretion.26 Secretin is released by acid in the duodenum and stimulates pancreatic fluid and bicarbonate secretion, leading to neutralization of acidic chyme in the intestine (see Chapter 56). Secretin also inhibits gastric acid secretion (see Chapter 49) and intestinal motility.
Human secretin is a 27–amino acid peptide and, similar to many other GI peptides, is amidated at the carboxyl terminus. It is the founding member of the secretin-glucagon-VIP family of structurally related GI hormones. Secretin is selectively expressed in specialized enteroendocrine cells of the small intestine called S cells.27
One of the major physiological actions of secretin is stimulation of pancreatic fluid and bicarbonate secretion (see Chapter 56). Pancreatic bicarbonate, on reaching the duodenum, neutralizes gastric acid and raises the duodenal pH, thereby “turning off” secretin release (negative feedback). It has been suggested that acid-stimulated secretin release is regulated by an endogenous intestinal secretin-releasing factor.28 This peptide stimulates secretin release from S cells until the flow of pancreatic proteases is sufficient to degrade the releasing factor and terminate secretin release.
Although the primary action of secretin is to produce pancreatic fluid and bicarbonate secretion, it is also an enterogastrone, a substance that is released when fat is present in the GI lumen and that inhibits gastric acid secretion. In physiologic concentrations, secretin inhibits gastrin release, gastric acid secretion, and gastric motility.29 The most common clinical application of secretin is in the diagnosis of gastrin-secreting tumors,30 as discussed in Chapter 32.
VASOACTIVE INTESTINAL POLYPEPTIDE
VIP is a neuromodulator that has broad significance in intestinal physiology. VIP is a potent vasodilator that increases blood flow in the GI tract and causes smooth muscle relaxation and epithelial cell secretion.31,32 As a chemical messenger, VIP is released from nerve terminals and acts locally on cells bearing VIP receptors. VIP belongs to a family of GI peptides, including secretin and glucagon, that are structurally related. The VIP receptor is a G protein–coupled receptor that stimulates intracellular cAMP generation.
Like other GI peptides, VIP is synthesized as a precursor molecule that is cleaved to an active peptide of 28 amino acids. VIP is expressed primarily in neurons of the peripheral-enteric and central nervous systems and is released along with other peptides, including primarily PHI and/or PHM (see Table 1-1).33
VIP is an important neurotransmitter throughout the central and peripheral nervous systems.34 Because of its wide distribution, VIP has effects on many organ systems; most notably, in the GI tract, VIP stimulates fluid and electrolyte secretion from intestinal epithelium and bile duct cholangiocytes.35,36
VIP, along with NO, is a primary component of nonadrenergic, noncholinergic nerve transmission in the gut.37 GI smooth muscle exhibits a basal tone, or sustained tension, caused by rhythmic depolarizations of the smooth muscle membrane potential. VIP serves as an inhibitory transmitter of this rhythmic activity, causing membrane hyperpolarization and subsequent relaxation of GI smooth muscle. Accordingly, VIP is an important neuromodulator of sphincters of the GI tract, including the lower esophageal sphincter and sphincter of Oddi. In certain pathologic conditions, such as achalasia and Hirschsprung’s disease, the lack of VIP innervation is believed to play a major role in defective esophageal relaxation and bowel dysmotility, respectively.38,39
Unlike GI endocrine cells that line the mucosa of the gut, VIP is produced and released from neurons and it is likely that most measurable VIP in serum is of neuronal origin. Normally, serum VIP levels are low and do not appreciably change with a meal. However, in pancreatic cholera, also known as Verner-Morrison syndrome and manifested by watery diarrhea, hypokalemia, and achlorhydria,40 VIP levels can be extraordinarily high.35 VIP-secreting tumors usually produce a voluminous diarrhea41 (see Chapter 32).
GLUCAGON
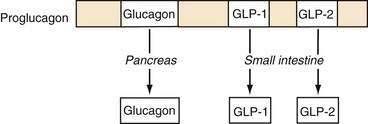
Glucagon is synthesized and released from pancreatic alpha cells and from intestinal L cells of the ileum and colon. Pancreatic glucagon is a 29–amino acid peptide that regulates glucose homeostasis via gluconeogenesis, glycogenolysis, and lipolysis and is counterregulatory to insulin. The gene for glucagon encodes not only preproglucagon but also glucagon-like peptides (GLPs). This precursor peptide consists of a signal peptide, a glucagon-related polypeptide, glucagon, and GLP-1 and GLP-2. Tissue-specific peptide processing occurs through prohormone convertases that produce glucagon in the pancreas and GLP-1 and GLP-2 in the intestine (Fig. 1-4).42
Glucagon and GLP-1 regulate glucose homeostasis.43 Glucagon is released from the endocrine pancreas in response to a meal and binds to G protein–coupled receptors on skeletal muscle and the liver to exert its glucoregulatory effects. GLP-1 stimulates insulin secretion and augments the insulin-releasing effects of glucose on the pancreatic beta cell (see later, “Enteroinsular Axis”). GLP-1 analogs have been developed for the treatment of type II diabetes mellitus. A long-acting human GLP-1 analog improves beta cell function and can lower body weight in patients with type II diabetes.44,45 GLP-2 is an intestinal growth factor and may have therapeutic implications in the maintenance of the GI mucosal mass and the reversal of villus atrophy.
GLUCOSE-DEPENDENT INSULINOTROPIC POLYPEPTIDE
GIP was discovered based on its ability to inhibit gastric acid secretion (enterogastrone effect) and was originally termed gastric inhibitory polypeptide. It was subsequently shown that the effects on gastric acid secretion occur only at very high concentrations that are above the physiologic range. However, GIP has potent effects on insulin release that (like GLP-1) potentiates glucose-stimulated insulin secretion.46 Based on this action, GIP was redefined as glucose-dependent insulinotropic polypeptide.
GIP receptors are also expressed on adipocytes through which GIP augments triglyceride storage, which may contribute to fat accumulation. Based on the insulinotropic properties of GIP, coupled with its effects on adipocytes, it has been proposed that GIP may play a role in obesity and development of insulin resistance associated with type II diabetes mellitus.47 Consistent with this proposal was the experimental finding that mice lacking the GIP receptor do not gain weight when placed on a high-fat diet.48 It remains to be seen whether GIP antagonists can be used to treat obesity. In rare circumstances, receptors for GIP may be aberrantly expressed in the adrenal cortex, resulting in food-dependent Cushing’s syndrome.49,50
PANCREATIC POLYPEPTIDE FAMILY
Originally isolated during the preparation of insulin, pancreatic polypeptide (PP) is the founding member of the PP family.51 The PP family of peptides includes NPY and peptide tyrosine tyrosine (PYY), which were discovered because of the presence of a C-terminal tyrosine amide.52,53 PP is stored and secreted from specialized pancreatic endocrine cells (PP cells),54 whereas NPY is a principal neurotransmitter found in the central and peripheral nervous systems.55 PYY has been localized to enteroendocrine cells throughout the GI tract but is found in greatest concentrations in the ileum and colon.56
The PP-PYY-NPY family of peptides functions as endocrine, paracrine, and neurocrine transmitters in the regulation of a number of actions that result from binding to one of five receptor subtypes.57 PP inhibits pancreatic exocrine secretion, gallbladder contraction, and gut motility.58 PYY inhibits vagally stimulated gastric acid secretion and other motor and secretory functions.59 An abbreviated form of PYY lacking the first two amino acids of the normally produced 36 amino acid peptide, PYY3-36, has been shown to reduce food intake when administered to humans, indicating that intestinally released peptide may play a role in regulating meal size.60 NPY is one of the most abundant peptides in the central nervous system and, in contrast to PYY3-36, is a potent stimulant of food intake.61 Peripherally, NPY affects vascular and GI smooth muscle function.62
SUBSTANCE P AND THE TACHYKININS
Substance P belongs to the tachykinin family of peptides, which includes neurokinin A and neurokinin B. The tachykinins are found throughout the peripheral and central nervous systems, and are important mediators of neuropathic inflammation.63 Tachykinins, as a group, are encoded by two genes that produce preprotachykinin A and preprotachykinin B. Common to both is a well-conserved C-terminal pentapeptide. Transcriptional and translational processing produce substance P, neurokinin A, and/or neurokinin B, which are regulated in large part by alternative splicing. These peptides function primarily as neuropeptides. Substance P is a neurotransmitter of primary sensory afferent neurons and binds to specific receptors in lamina I of the spinal cord.64 Three receptors for this family of peptides have been identified—NK-1, NK-2, and NK-3.65 Substance P is the primary ligand for the NK-1 receptor, neurokinin A for the NK-2 receptor, and neurokinin B for the NK-3 receptor. However, all these peptides can bind and signal through all three receptor subtypes.
Substance P has been implicated as a primary mediator of neurogenic inflammation. In the intestine, Clostridium difficile–initiated experimental colitis results from toxin-induced release of substance P and consequent activation of the NK-1 receptor.66 These inflammatory sequelae can be blocked by substance P receptor antagonists. Substance P receptors are more abundant in the intestine of patients with ulcerative colitis and Crohn’s disease.67
SOMATOSTATIN
Somatostatin is a 14–amino acid cyclic peptide that was initially identified as an inhibitor of growth hormone secretion. Since its discovery, it has been found in almost every organ in the body and throughout the GI tract. In the gut, somatostatin is produced by D cells in the gastric and intestinal mucosa and islets of the pancreas, as well as enteric neurons.68 Somatostatin has a number of pharmacologic effects that are mostly inhibitory.
In the stomach, somatostatin plays an important role in regulating gastric acid secretion.69 In the antrum, D cells are open to the lumen, where they are directly exposed to acid. A low gastric pH stimulates D cells that lie in close proximity to gastrin-producing cells to secrete somatostatin and inhibit gastrin release (see Chapter 49). Reduced gastrin secretion decreases the stimulus for acid production and the pH of the stomach contents rises. Thus, some of the inhibitory effects of gastric acid on gastrin release (see earlier, “Gastrin”) are mediated by somatostatin.
Somatostatin release is also influenced by mechanical stimulation, dietary components of a meal, including protein, fat, and glucose, and other hormones and neurotransmitters.70 Muscarinic stimulation appears to be the most important neural stimulus to somatostatin secretion.
At least five somatostatin receptors have been identified that account for divergent pharmacologic properties.71 For example, receptor subtypes 2 and 3 couple to inhibitory G proteins but receptor subtype 1 does not. In addition, only somatostatin receptor subtype 3 inhibits adenylate cyclase. The inhibitory effects of somatostatin are mediated by a decrease in cAMP, Ca2+ channel inhibition, or K+ channel opening.
In the gut, somatostatin has broad inhibitory actions. In addition to effects on gastric acid, somatostatin reduces pepsinogen secretion. Somatostatin profoundly inhibits pancreatic enzyme, fluid, and bicarbonate secretion and reduces bile flow.72 The effects of somatostatin on gut motility are largely inhibitory, with the exception that it stimulates the migrating motor complex, possibly through effects on motilin. Somatostatin also reduces intestinal transport of nutrients and fluid, reduces splanchnic blood flow, and has inhibitory effects on tissue growth and proliferation.73,74
Because of its varied physiologic effects, somatostatin has several clinically important pharmacologic uses. Many endocrine cells possess somatostatin receptors and are sensitive to inhibitory regulation. Therefore, somatostatin and more recently developed somatostatin analogs are used to treat conditions of hormone excess produced by endocrine tumors, such as acromegaly, carcinoid tumors, and islet cell tumors (including gastrinomas).75 Its ability to reduce splanchnic blood flow and portal venous pressure has led to somatostatin analogs being useful in treating esophageal variceal bleeding (see Chapter 90).76 The inhibitory effects on secretion have been exploited by using somatostatin analogs to treat some forms of diarrhea and reduce fluid output from pancreatic fistulas. Many endocrine tumors express abundant somatostatin receptors, making it possible to use radiolabeled somatostatin analogs, such as octreotide, to localize even small tumors throughout the body.
MOTILIN
Motilin is a 22–amino acid peptide produced by endocrine cells of the duodenal epithelium.77 Motilin is secreted into the blood in a periodic and recurrent pattern that is synchronized with the migrating motor complex (MMC) under fasting conditions. Elevations in blood motilin levels regulate the phase III contractions that initiate in the antroduodenal region and progress toward the distal gut. Motilin secretion is not stimulated by eating.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree