CHAPTER 33 Gastrointestinal Consequences of Infection with Human Immunodeficiency Virus
For the 15 years of the acquired immunodeficiency syndrome (AIDS) epidemic that antedated effective antiviral therapy for the human immunodeficiency virus (HIV), the world witnessed an explosion of cases typically manifested by opportunistic infections (e.g., Pneumocystis jiroveci pneumonia) and neoplasms (e.g., Kaposi’s sarcoma). Early in the epidemic, the focus of attention was on characterizing these disorders, and when effective, using prophylactic antimicrobial therapy. In 1995 the concept of highly active antiretroviral therapy (HAART) was born, and the face of the epidemic changed overnight. HAART decreases viral replication and, consequently, circulating HIV. In some patients HIV becomes undetectable in the blood. Associated with a reduction in viral load, there is substantive improvement in immune function that can be assessed by objective measures such as an increase in the CD4 lymphocyte count and clinically by a decrease in opportunistic infections (OIs), as well as improved survival.1–3 With immune reconstitution provided by HAART, both primary and secondary prophylaxis against a variety of OIs also may be discontinued.4 The current focus of management thus centers around viral control rather than prevention and treatment of opportunistic infections. Although dramatic changes have been witnessed as a result of HAART, the global epidemic continues, ensuring that this disease will be present for years to come.5
With the immune reconstitution associated with HAART, there also has been a shift to the management of chronic diseases, as well as drug side effects. Hepatitis C virus (HCV) infection is highly prevalent in HIV-infected patients. Because of HAART, chronic liver disease has assumed increasing importance, as evidenced by reports demonstrating that end-stage liver disease, most often a result of HCV, is a leading cause of hospitalization and death.6 Similarly, HIV-infected patients responding to HAART who have gastrointestinal (GI) complaints are more likely to have drug-induced side effects or nonopportunistic gastrointestinal infections, shifting management strategies back to disorders prevalent in normal hosts.7
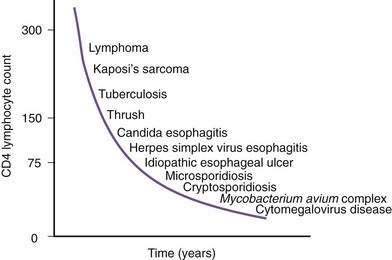
Figure 33-1. Time line of opportunistic disorders based on CD4 lymphocyte count.
(From Wilcox CM, Saag MS. Gastrointestinal complications of HIV infection: Changing priorities in the HAART era. Gut 2008; 57: 861-70.)
ODYNOPHAGIA AND DYSPHAGIA
Before the era of HAART, esophageal complaints (dysphagia and odynophagia) were commonly reported to occur in at least one third of patients during the course of HIV disease. Because of HAART, the incidence of esophageal disease has fallen, and the number of patients with diseases not unique to AIDS, such as gastroesophageal reflux, has risen.7
Candida albicans, the most frequent esophageal infection in AIDS, frequently coexists with other disorders in this setting. Although most cases of Candida occur in the setting of AIDS, Candida esophagitis may occur during primary HIV infection as a result of transient immunosuppression.8 Oral thrush often predicts concurrent esophagitis; however, the absence of thrush does not exclude the possibility of esophageal candidiasis. Overall, the positive and negative predictive values of thrush for Candida esophagitis for patients naïve to HAART are 90% and 82%, respectively.9
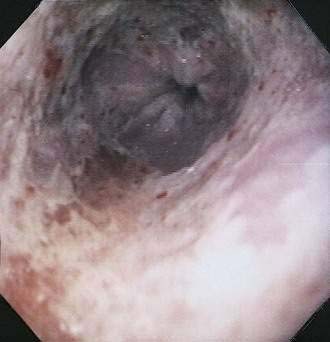
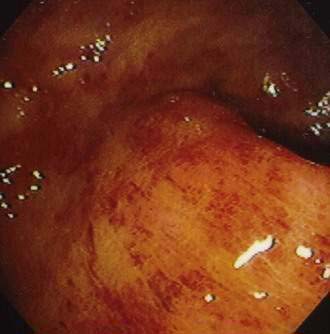
Although CMV is the most commonly identified pathogen in AIDS, its association with esophageal disease is less frequent than Candida. CMV causes mucosal ulceration; thus patients with CMV esophagitis complain of odynophagia or substernal chest pain, characteristically severe.10 Dysphagia is much less common than in patients with Candida esophagitis and is rarely the primary complaint. Fever is rare. Generally, upper endoscopy reveals extensive ulcerations that are large and deep, although the endoscopic pattern is variable (Fig. 33-2).11 Candidal coinfection is common. Mucosal biopsies characteristically demonstrate viral cytopathic effect in mesenchymal and/or endothelial cells in the granulation tissue. As is typical for gut involvement with CMV, characteristic inclusions may be absent, necessitating confirmation by immunohistochemical stains. Biopsy of granulation tissue in the ulcer base provides the highest yield for viral cytopathic effect, whereas viral culture is less sensitive and cytologic brushings are unhelpful.12
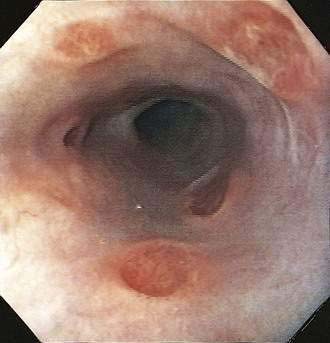
A syndrome of nonspecific (idiopathic, aphthous) esophageal ulceration is common (Fig. 33-3).10 The clinical presentation and endoscopic appearance are indistinguishable from CMV. Criteria for diagnosis of idiopathic ulcers include the following: (1) endoscopic and histopathologic ulcer; (2) no evidence of viral cytopathic effect by both routine histology and immunohistochemical studies; and (3) no clinical or endoscopic evidence of reflux disease or pill-induced esophagitis. As with CMV, these ulcers occur in late-stage disease, with most patients having a CD4 count less than 50/mm3. However, they have also been described in patients with the acute HIV seroconversion syndrome. The pathogenesis of these ulcers remains unknown, but mucosal HIV infection does not appear to be causative.
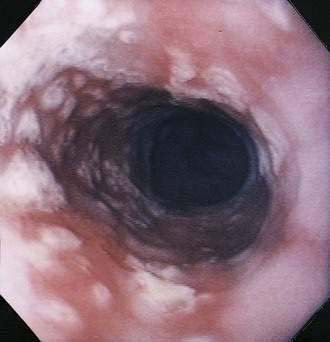
In contrast with other immunocompromised hosts, herpes simplex virus (HSV) esophagitis is infrequent in AIDS.10 In immunocompetent patients, esophagitis is usually due to HSV type 1; however, AIDS patients may have esophagitis due to either type 1 or type 2 herpes. The disease is similar to herpetic infections of other mucous membranes in that the pathogenetic features follow a predictable sequence: discrete vesicles form, then shallow ulcers, which finally coalesce into regions of diffuse shallow ulceration. It is during this late stage of diffuse esophagitis that most patients with herpes are evaluated. In contrast with CMV esophagitis and idiopathic ulcer, these ulcers tend to be shallow; large, deep ulcers are rare (Fig. 33-4). Biopsies and cytologic brushings taken from the margin of the ulcers (the sites of active viral replication) are most likely to show epithelial cell invasion and nuclear changes typical of herpes infections. Viral cultures of biopsy specimens are usually positive.12
EVALUATION AND MANAGEMENT
A specific cause of esophageal complaints in the AIDS patient cannot be made on the basis of symptoms or physical examination alone (Table 33-1). Nevertheless, a few generalizations may be made. The presence of oral thrush associated with mild to moderate dysphagia without odynophagia is likely caused by Candida esophagitis. In contrast, the patient with severe odynophagia without dysphagia or thrush is more likely to have ulcerative esophagitis (viral, idiopathic). The patient complaining of substernal burning and regurgitation is most likely to have gastroesophageal reflux disease. Intermittent solid and liquid dysphagia may be related to a motility disturbance.13
Table 33-1 Differential Diagnosis of Dysphagia and Odynophagia in Patients with AIDS
AIDS, acquired immunodeficiency syndrome.
Endoscopy with biopsy is the only means of establishing a specific etiology for the cause of dysphagia and odynophagia. Conventional barium swallow radiography in the patient with esophageal complaints is not worthwhile, although it may reveal typical features of Candida, CMV, or herpes. In addition, the radiographic appearance of an esophageal ulcer cannot adequately distinguish etiology, thus mandating subsequent endoscopy and biopsy for a definitive diagnosis. Multiple mucosal biopsies are preferred over brush cytology for ulcerated lesions.12
Given the preponderance of Candida infection, an empirical approach to the management of esophageal symptoms is reasonable in most patients with AIDS. Patients with dysphagia and/or odynophagia who also have oral thrush should be treated empirically with fluconazole 100 mg/day after a 200-mg loading dose.14 Itraconazole or fluconazole suspensions are effective alternatives. If symptoms persist despite a 1-week empirical trial, endoscopy with biopsy should be performed in preference to the initiation of other empirical trials or escalation of the dose of fluconazole. Narcotics are appropriate for the patient with severe pain until specific treatment for the underlying cause can be initiated. Relapse of Candida esophagitis is invariable unless immune function is improved with antiretroviral treatment. Despite chronic prophylaxis, relapse frequently occurs often due to antifungal resistance. This is much less of a problem now that patients are receiving HAART. In the absence of HAART, caspofungin and posaconazole may be effective for patients with resistance to antifungal drugs.15
CMV and HSV infection should be treated similarly to other gut involvement with these viruses (see following). Idiopathic ulcers respond in more than 90% of patients to oral glucocorticoids (e.g., 40 mg prednisone per day initially, tapered over 4 weeks).16 The basis for glucocorticoid efficacy is unknown; infectious causes should be assiduously excluded before administering gluocorticoids in this setting. Thalidomide is also highly effective and may be curative when prednisone fails.16 The main side effects of thalidomide are somnolence, rash, and neuropathy. The devastating teratogenic effects mandate its use to be limited to men.
DIARRHEA
Before HAART, diarrhea occurred in up to 90% of patients during the course of HIV disease, especially those from developing countries. In the era of HAART, diarrhea is a less frequent complaint and etiologically is now most often drug-induced (antiretroviral therapy) or is caused by disorders unrelated to HIV infection.17 Alterations in the mucosal immune system in AIDS predispose to intestinal infections, may lead to untreatable chronic infection by organisms that typically cause self-limited infection in healthy hosts (e.g., Cryptosporidium), and may contribute to a more virulent clinical course of otherwise common enteric infections (e.g., Salmonella, Shigella, Campylobacter). Despite the vast spectrum of protozoal, viral, bacterial, and fungal organisms that cause diarrhea in the patient with AIDS, a differential diagnosis can be developed on the basis of the clinical presentation and degree of immunodeficiency (Table 33-2).
Table 33-2 Differential Diagnosis of Diarrhea in Patients with AIDS
| Protozoa |
| Bacteria |
| Viruses |
| Fungi |
| Neoplasms |
| Idiopathic |
| Drug Induced |
| Pancreatic Disease |
AIDS, acquired immunodeficiency syndrome; CMV, cytomegalovirus; HIV, human immunodeficiency virus.
EVALUATION AND MANAGEMENT
Protozoa account for the most prevalent class of diarrheal pathogens in most series,18 largely because many of these infections can lead to chronic diarrhea and are refractory to treatment. Cryptosporidium, a cause of self-limited diarrhea in healthy hosts, remains the most frequent protozoa identified in HIV-infected patients worldwide.18 Clinical presentation and outcome are related to the degree of immunocompromise and the subtype of organism.19 Stool testing of asymptomatic individuals shows a high carriage rate because some subtypes appear to be less pathogenic.20 The small bowel is the most common site of infection, although the organisms can be recovered in all regions of the gut, as well as in biliary and respiratory epithelium. Diarrhea is typically severe, with stool volumes of several liters per day not uncommon. Borborygmi, nausea, and weight loss are frequently associated symptoms; right upper quadrant pain suggests biliary tract involvement (see later). The pathogenesis of this infection is uncertain. The diagnosis of intestinal cryptosporidiosis is most often made by acid-fast stain of the stool, where the organisms appear as bright red spherules, similar in size to red blood cells. The sensitivity of stool testing varies and depends on the burden of organisms, character of the stool (formed versus liquid), and primary site of infection. Stool antigen detection and polymerase chain reaction (PCR) markedly increase sensitivity of stool testing. Cryptosporidia may be identified in small bowel or rectal biopsies even when the stool examination is negative.21
Specific antimicrobial treatment of cryptosporidial infection remains disappointing. Numerous antimicrobial agents have been tested, most without significant effect (Table 33-3). Nitazoxanide and azithromycin have been most recently evaluated with mixed results, and combination therapies have also been used. Nitazoxanide can lead to cryptosporidial oocyte clearance in HIV-seronegative patients but has not shown efficacy in HIV-infected patients.22 Although not pathogen-specific, currently the most effective therapy for cryptosporidia is HAART, in which improvement of immune function results in a clinical remission of diarrhea and clearance of cryptosporidia from the stool and on small bowel biopsy.23 For patients failing HAART and/or in whom antimicrobial therapy is ineffective, symptomatic treatment should include fluid support, antidiarrheal agents, and occasionally narcotics such as tincture of opium to control the diarrhea.
Table 33-3 Treatment of Infectious Causes of Diarrhea in Patients with AIDS
| PATHOGEN | TREATMENT | DURATION (DAYS) |
|---|---|---|
| Protozoa | ||
| Cryptosporidia | Paromomycin, azithromycin, nitazoxanide | 14-28 |
| Cyclospora spp. | Trimethoprim-sulfamethoxazole or ciprofloxacin | 14-28 |
| Isospora belli | Trimethoprim-sulfamethoxazole or ciprofloxacin or pyrimethamine | 14-28 |
| Microsporidia | Albendazole (Encephalitozoon intestinalis) | 14-28 |
| Metronidazole, atovaquone, fumagillin (not available in United States)† | ||
| Viruses | ||
| Cytomegalovirus | Ganciclovir | 14-28* |
| Foscarnet | 14-28* | |
| Cidofovir | 14-28* | |
| Herpes simplex | Acyclovir | 5-10* |
| Bacteria | ||
| Salmonella, Shigella, Campylobacter spp. | Fluoroquinolone (e.g., ciprofloxacin) | 10-14* |
| Clostridium difficile | Vancomycin, metronidazole | 10-14 |
| Small intestinal bacterial overgrowth | Metronidazole, ciprofloxacin | 10-14 |
| Mycobacterium tuberculosis | Isoniazid, rifampin, pyrazinamide, ethambutol | 9-12 mo |
| Mycobacterium avium complex | Multidrug regimens for symptomatic infection (see text) | 9-12 mo |
| Fungi | ||
| Histoplasmosis | Amphotericin B; then itraconazole | 28 |
| Coccidioidomycosis | Amphotericin B; then fluconazole | 28 |
| Cryptococcosis | Amphotericin B; then fluconazole | 28 |
AIDS, acquired immunodeficiency syndrome.
* Duration of therapy dictated by immune reconstitution with highly active antiretroviral therapy.
† Molina JM, Tourneur M, Sarfati C, et al. Fumagillin treatment of intestinal microsporidiosis. N Engl J Med 2002; 346:1963.
Isospora belli is a sporozoan, which, like Cryptosporidium, is a cause of chronic diarrhea in untreated patients with HIV infection. The disease is rare in the United States, but it is more frequent and endemic in developing countries such as Haiti. The organism may be identified by acid-fast stain of the stool or duodenal secretions or on mucosal biopsy. This infection can be effectively treated with antibiotics, specifically trimethoprim-sulfamethoxazole and ciprofloxacin.24
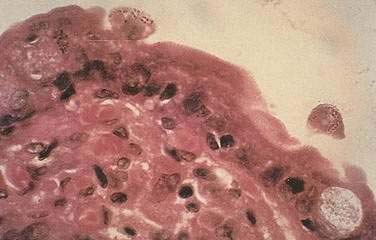
Microsporidia emerged as common intestinal infections in AIDS, but their prevalence has markedly fallen in the HAART era.25 Intestinal and hepatobiliary disease may be caused by two species of microsporidia: Enterocytozoon bieneusi and Encephalitozoon intestinalis. The reported prevalence of microsporidia without HAART varied from 15% to 39%.18,21,26 Typical symptoms include watery, nonbloody diarrhea of mild to moderate severity usually without associated crampy abdominal pain. Weight loss is common, although not to the degree observed with Cryptosporidium. Infection is associated with severe immunodeficiency with median CD4 counts of infected individuals of less than 100/mm3.26 As with infection from cryptosporidia, the pathogenesis of disease remains poorly defined. The organism incites little tissue inflammation and is rarely associated with villous atrophy and cell degeneration. Microsporidia can be discerned by light microscopy when tissue is embedded in plastic or paraffin (Fig. 33-5). Staining of embedded mucosal biopsies with Brown-Brenn, Gram stain, or modified Masson trichrome stain is superior to routine hematoxylin and eosin staining.27 E. intestinalis can usually be differentiated from E. bieneusi by its larger size and infection of lamina propria macrophages; electron microscopy is definitive. Stool staining techniques are only moderately sensitive, while small bowel biopsies are generally positive. No effective therapy is available for E. bieneusi, whereas albendazole is effective for E. intestinalis.28 As with the treatment of cryptosporidia, HAART is the best therapy, resulting in resolution of diarrhea with loss of this pathogen from stool and on small bowel biopsy.23
For unclear reasons, infections by the protozoa Giardia lamblia and Entamoeba histolytica are not consistently seen with increased frequency or virulence in AIDS.29 However, in a study from Taiwan, where E. histolytica is endemic, amebic colitis was identified as a common cause of diarrhea.30 The nonpathogenic Entamoeba dispar is morphologically similar to E. histolytica and can only be distinguished by more specific stool or enzyme-linked immunosorbent assay tests.29 Blastocystis hominis, Endolimax nana, and Entamoeba coli are nonpathogenic protozoa that are seen more commonly in men who have sex with men and are often found in association with other protozoal parasites. Rare cases of enteric leishmaniasis, Pneumocystis jiroveci infection, and toxoplasmosis have been reported.
Helminths, particularly Strongyloides stercoralis and Ascaris lumbricoides, are uncommon pathogens.31 Patients may present with abdominal pain, diarrhea, and eosinophilia. The clinical syndrome and recurrence rate associated with these parasites do not appear to be altered in the setting of HIV infection.
Viral infection of the large bowel, and rarely the small bowel, is an important cause of diarrhea in HIV infection. CMV is the most common viral cause of diarrhea and the most frequent cause of chronic diarrhea in patients with AIDS and multiple negative stool tests.32 This infection characteristically occurs late in the course of HIV infection when the CD4 lymphocyte count falls below 100/L (see Fig. 33-1). Infection is most common in the colon (Fig. 33-6), but concomitant disease in the esophagus, stomach, or small bowel may be observed. Isolated small bowel disease typically results in abdominal pain rather than diarrheal illness. The pathogenesis has not been totally elucidated. Infection of vascular endothelial cells is common, suggesting a role for mucosal ischemia; true histopathologic evidence of vasculitis is rare. An important role for local proinflammatory cytokine activation has been suggested.33
The clinical manifestations of enteric CMV infection vary greatly and include asymptomatic carriage, nonspecific symptoms of weight loss and fevers, and focal enteritis/colitis including appendicitis or diffuse ulcerating hemorrhagic involvement with bleeding or perforation. As a result, patients can present with one of several constellations of symptoms, including abdominal pain; peritonitis; watery, nonbloody diarrhea; or hematochezia.34 The most common presentation, however, is abdominal pain associated with chronic diarrhea. Although the endoscopic spectrum is variable, the hallmark of CMV enteritis/colitis is subepithelial hemorrhage and mucosal ulceration (see Fig. 33-6).34
The diagnosis of GI CMV infection is best established by demonstrating viral cytopathic effect in tissue specimens.35 The inclusions may be atypical in appearance or few in number, requiring immunostaining and/or in situ hybridization for confirmation.35 Cultures for CMV are usually positive when inclusions are present, but they are less sensitive and specific for CMV infection than histopathologic identification. If inclusions are few in number and are demonstrable in tissue that appears macroscopically normal, the patient should be considered to have CMV colonization rather than true CMV infection.
A number of effective therapies are available for the treatment of CMV (see Table 33-3). The most commonly used agent is ganciclovir, an acyclovir derivative, which is effective in approximately 75% of cases.36 Ganciclovir requires daily intravenous administration for several weeks, depending on the location and severity of disease. Valganciclovir, an oral analog of ganciclovir, has excellent GI absorption and efficacy for CMV retinitis, but has not been well studied for induction therapy in GI disease. Valganciclovir has become widely used as preemptive therapy in the transplant setting and has shown promise as first-line therapy.37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree