Gastrointestinal Bleeding: A Multidisciplinary Approach
Gastrointestinal (GI) hemorrhage results in 250,000 to 300,000 U.S. hospital admissions each year, with bleeding due to peptic ulceration accounting for a significant number of these admissions.1,2 The epidemiologic aspect of GI bleeding is complex and dynamic:3–5 gastric ulcer rates have increased over the past several decades in part due to the common use of nonsteroidal anti-inflammatory drugs (NSAIDs).6–8 Furthermore, Helicobacter pylori infection rates also increase with age. Although not yet fully understood, the importance of H. pylori in ulcer pathogenesis is now widely recognized.9, Lastly, vascular anomalies, such as angiodysplasias, are recognized more often as a source of upper, small intestinal, and lower GI bleeding.10 These are often multiple and bleed intermittently, posing added challenges to their diagnosis and management.
Despite improved medical and surgical care, the overall 6 to 7% mortality for GI hemorrhage has not improved in more than two decades, possibly because of the comorbidity of our increasingly elderly population.1,5 Limitations in medical therapy for actively bleeding ulcers helped pave the road for advancements in fiberoptic technology such that by the early 1980s it was routine practice to perform early endoscopy in bleeding patients.11,12 Over the last decade and a half, endoscopic therapeutic applications for patients with active
bleeding or visible vessels at endoscopy (including injection therapy, laser, and thermal coagulation) have resulted in reduced hopital stays, decreased transfusion requirements, significantly reduced bleeding and rebleeding, and decreased need for emergency surgery.11 Meta-analysis of endoscopic hemostatic therapies for nonvariceal upper GI hemorrhage also confirms a significant reduction in mortality.13 Surgical laparoscopists are now able to apply minimally invasive surgery for the treatment of small bowel and gastric lesions that are not amenable to endoscopic methods.14,15 The appropriate use of interventional radiologic techniques has also complemented the diagnosis and definitive management of many bleeding GI lesions.16–19
In many instances, choosing a gastroenterologic, surgical, or radiologic approach to GI bleeding is determined by local expertise and experience. The clinical impact of managed care and the adoption of practice guidelines may also be influential. More than ever before, clinical gatekeepers must be familiar with the specific utility and the predictive value of diagnostic tests and therapeutic procedures in their bleeding patients. Finally, more studies comparing costs, follow-up care, and long-term outcome are needed to clarify which techniques or therapies are most appropriate for the individual patient.
UPPER GASTOINTESTINAL BLEEDING
Medical Resuscitation
Because an estimated 70 to 80% of GI bleeding stops spontaneously, the clinician’s primary objective is to prevent damage to vital organs from hypoperfusion.20 Bleeding from upper GI sources may be obvious when the patient presents with hematemesis, or occult in cases of severe iron deficiency anemia and Hemoccult-positive stool. Historical clues, including ulcer-type pain, use of NSAIDs, or underlying renal disease (risk for angiodysplasias) or liver disease (risk for portal hypertension), may be helpful. The presence of orthostatic hypotension indicates about 20% blood volume loss, whereas resting tachycardia, shock, azotemia, or lactic acidosis indicates major blood loss and need for intensive care.21 The placement of a nasogastric (NG) tube can be helpful and predictive of outcome. Aspirating “coffee ground” material may help establish the upper GI tract as the source of bleeding. Obtaining fresh persistently red blood that does not “clear” with continued lavage is particularly ominous because the mortality increases to as high as 50% in such patients.22,23 Caution is advised not to overinterpret a negative NG aspirate because the tube may be malpositioned in the esophagus or proximal stomach and therefore not representative of more distal contents. Aspiration of clear greenish bile–colored fluid indicates an adequate sample in a nonacutely bleeding patient but does not predict the chance of re-bleeding. There is a generally recognized higher mortality from upper GI hemorrhage in elderly patients due to comorbidity and a higher likelihood of there being deeper ulcers in the gastric body.24 Although controversial, there is no consensus on whether NSAID use as a cause of bleeding is associated with higher mortality compared with other causes of bleeding peptic ulcer.25
Effective resuscitative measures include the prompt establishment of intravenous access and administration of fluids and blood products. The formerly routine administration of fresh frozen plasma as well as packed red blood cells for patients with ongoing hemorrhage is outdated. However, patients with known specific factor deficiencies, cirrhotic patients,26 and anticoagulated patients27 may require plasma or specific replacement factors. Patients with altered mental status or who repeatedly vomit blood are at high risk for aspiration. Therefore, elective endotracheal intubation should be considered in these patients if urgent endoscopy is necessary for bleeding control.28 Administration of the fluoroquinolone antibiotic norfloxacin in cirrhotic patients with acute bleeding has been shown to reduce infectious complications.29 Pharmacologic treatment for the control of bleeding should be begun as early as possible, when indicated.
While resuscitating, clinicians should consult endoscopists, surgeons, and interventional radiologists for their collaborative input and subsequent management. About 20% of patients with upper GI hemorrhage will have continuous or intermittent bleeding. These are the patients with the highest overall mortality, and shock at presentation is the best clinical predictor of recurrent hemorrhage.30
Pharmacologic Control of Bleeding
Control of Acute Bleeding
Formerly, intravenous pitressin with or without nitroglycerin was used to control hemorrhage from esophageal and gastric varices. Pitressin causes splanchnic arteriolar constriction, resulting in 20 to 30% decreased portal and azygous venous pressure.31 Nitroglycerin decreases splanchnic blood flow via venodilatation; its recommended use with pitressin is to combat the systemic side effects of coronary, renal, and mesenteric artery constriction, increased peripheral vascular resistance, and potential for ischemia.32 Popular in the last decade, intravenous octreotide, a synthetic somatostatin analogue, has been proven effective and safe. Its mechanism of action, though not completely understood, includes directly increasing the pressure of the lower esophageal sphincter (tamponade effect) and reducing blood flow to the adjacent vasculature. Systemic effects include lowering of splanchnic pressure without increase in peripheral vascular resistance. Studies of octreotide alone and as an adjunct to sclerotherapy or band ligation of varices reveal its clinical usefulness in acutely controlling portal hypertensive bleeding.33,34 A recent meta-analysis of the use of octreotide or somatostatin in hospitalized patients with endoscopically proven non-variceal-related upper GI hemorrhage found that treated patients were significantly less likely to rebleed or require surgery.35
Prevention of Portal Hypertensive Bleeding
Prophylactic therapy for the prevention of recurrent hemorrhage associated with portal hypertension cannot be overemphasized. Patients with esophageal and gastric varices or portal hypertensive gastropathy should be maintained on a noncardioselective (3-blocker titrated to reduce the resting heart rate by 25%, which causes about a 25 to 30% reduction in portal pressure. Propranolol reduces the incidence of initial bleeding,36 increases the time between bleeding episodes,37 and is superior to sclerotherapy for the prevention of first bleeding.38 It reduces heart rate and renal blood flow as well as portal pressure but is metabolized in the liver, so that metabolism may be erratic in cirrhotic patients. Common side effects include fatigue, sleep disturbances, and changes in libido, all limiting medical compliance. Nadolol, another noncardioselective (3-blocker, also prevents first episodes of bleeding.39,40 Because this drug does not affect renal blood flow and is not metabolized by the liver, it may emerge as the preferred (3-blocker for chronic pharmacologic reduction of portal pressure. Many clinicians add nitrates to (3-blocker therapy for added portal pressure reduction, or as single therapy for patients who are intolerant of (3-blockers.41 Experimentally, nitrates reduce mesenteric blood flow by venodilatation, resulting in a baroreceptor-mediated compensatory arterial vasoconstriction.42 Nitrates may also reduce portal pressure by relaxation of the presinusoidal and septal myofibroblasts in the liver, resulting in decreased resistance to portal flow43,44 Hemodynamic studies of cirrhotic patients have demonstrated decreases in mean arterial pressure and in the hepatic vein pressure gradient with organic nitrates.45–47 A study comparing (3-blocker plus nitrates with nitrates alone revealed a greater reduction in the hepatic vein pressure gradient with combination therapy48 Whether the observed greater hemodynamic effect reduces the rate of rebleeding awaits the results of randomized clinical trials. Clonidine, a centrally acting a2agonist, has been shown to decrease portal pressure by decreasing postsinusoidal resistance and constricting the splanchnic circulation.49 Controlled trials have not been performed comparing the efficacy of clonidine over (3-blockers or nitrates. In a recent small randomized study of patients with biopsy-proven cirrhosis and variceal hemorrhage, the group receiving twice-daily subcutaneous injections of octreotide plus routine sclerotherapy had decreased mortality, decreased rebleeding, lower hepatic venous pressure, and overall improved hepatic function compared with the group that received sclerotherapy alone.50
Acid-Suppressive Therapy
Chronic acid suppression for maintaining remission of upper GI bleeding is indicated for erosive esophagitis or esophageal ulcers. Proton pump inhibitors are clinically and economically superior to H2 receptor antagonists for acute and chronic healing of erosive esophagitis.51 Proton pump inhibitors are superior to all other agents in reducing recurrent bleeding and ulceration associated with NSAID-induced gastric or duodenal ulcers.52 They are recommended for any patient with a history of bleeding from NSAID-associated ulceration who continues to use these medications. Chronic acid suppression is recommended for patients with non-NSAID-associated bleeding who are not infected with H. pylori. In this group, representing less than 10% of ulcer patients, hypersecretory conditions should be excluded. Critically ill patients requiring mechanical ventilation have an increased risk of stress gastritis and should be treated prophylactically with acid-suppressive medicines.53
Helicobacter Pylori Eradication
At least 90% of non-NSAID-induced peptic ulcers are associated with H. pylori infection. Successful eradication of this organism results in significantly reduced rates of both recurrent ulceration9,54 and recurrent bleeding.55–57 Repeat upper endoscopy with biopsy or urease breath testing is justified in patients with prior H. pylori-induced bleeding ulcers to ensure eradication.58 In 1992, a Consensus Development Conference of the National Institutes of Health recommended that all patients with ulcers who are infected with H. pylori receive antimicrobial therapy.59 It will likely take decades before the impact of this recommendation is realized; it is predicted that the cost of H. pylori eradication will compare favorably with that of chronic acid suppression or surgery.
Endoluminal Management
Diagnostic Endoscopy
Traditionally, the term “upper GI bleeding” refers to bleeding mucosal lesions proximal to the ligament of Treitz. In most instances, standard upper endoscopic procedures expose very little of the hypopharyngeal mucosa, distal duodenum, or proximal jejunum. A carefully obtained bleeding history should allow the endoscopist to select the appropriate equipment, with an expected endoscopic diagnostic yield of 90 to 95%. Occasionally, retained blood clots obscure the bleeding site. Prior evacuation of the stomach with a large-bore Ewald or Edlich tube is often helpful. Patients can also be turned from the standard left lateral position to shift retained blood clots to more gravity-dependent areas. Lesions likely to be missed under fundic clots include Mallory-Weiss tears, gastric varices, Dieulafoy’s lesion (a type of arteriovenous malformation), and malignancies. Peptic ulcer disease predictably causes ulcerations in the duodenal bulb, the immediate postbulbar region, the gastric antrum, and the lesser curvature of the gastric body. Even when the stomach is full of retained fluid and clots, these areas can usually be thoroughly examined. Nodular antral mucosa with patchy erythema may clue the endoscopist to the possibility of H. pylori infection, and a biopsy can be obtained at endoscopy for the presence of urease.60 Esophageal varices are usually readily apparent; stigmata of recent bleeding include cherry-red spots and red whale markings. Gastric varices are often confused with prominent rugal folds, making their distinction less definite during endoscopy. About 50% of upper GI bleeding is due to peptic ulcer, and about 15% is from esophageal or gastric varices, with esophagitis, erosive duodenitis, Mallory-Weiss tears, portal hypertensive gastropathy, and vascular anomalies accounting for most of the remainder.1,10,61,62 More obscure causes, such as Dieulafoy’s lesion, watermelon stomach, duodenal varices, and pancreaticobiliary sources account for less than 2% of cases.63–67
When standard upper endoscopy fails to identify a bleeding site despite a history suggestive of upper GI bleeding, another endoscopy can be performed with a longer instrument. Adult or pediatric colonoscopes can usually be inserted to or just beyond the ligament of Treitz; small bowel push enteroscopes with or without an overtube can reach up to 1 m beyond the ligament of Treitz.68,69 When the distal jejunum or proximal ileum warrants visualization, the sonde (pull) enteroscope may be useful. This instrument is 3 m long and 5 mm in diameter, allowing for transnasal (or transoral) passage. The entire small bowel can be traversed in as little as 4 to 6 hours. An estimated 50 to 75% of small intestinal mucosa is surveyed during the viewing (pulling back) portion of the examination.70,71 Unlike all of the other modern endoscopes, therapeutic capabilities with the sonde have not been developed. The instrument tip can be deflected, and a balloon can be inflated to slow down the removal so that plain film x-rays or video can be obtained for future reference. Small bowel push enteroscopes, including the sonde, can be used in conjunction with laparotomy or laparoscopy for localization of bleeding sites prior to planned surgical interventions.70 The diagnostic yield using these enteroscopes when standard endoscopy is unrevealing is 30 to 40%, and the main bleeding lesions encountered are vascular anomalies. Jejunal or ileal ulcers, bleeding diverticulae, and neoplasias are rarely found.70,71
Endoscopic Findings and Interventions Predict Outcomes
The endoscopic appearance of bleeding lesions is predictive of rebleeding rates, morbidity, mortality, and need for emergency surgery.72–80 Because ulcers with a yellow-white “clean” base have a less than 5% risk of rebleeding, patients with clean-based ulcers may be discharged safely after medical stabilization and treatment. Ulcers with flat red or black spots either within them or along their edges have a less than 10% incidence of rebleeding (Fig. 1-1). An adherent clot has a rebleeding potential of about 25% because the clot may overlie a flat lesion or a protruding vessel (Fig. 1-2). Controversy exists as to whether it is advisable to dislodge a clot. A visible vessel or pigmented protuberance created from a vessel with a small attached clot has a rebleeding rate of 50% (Figs. 1–3 and 1–4). Active bleeding at endoscopy carries the highest rate of rebleeding at 70 to 80%. Mortality in patients with recurrent bleeding may be as high as 40%.81
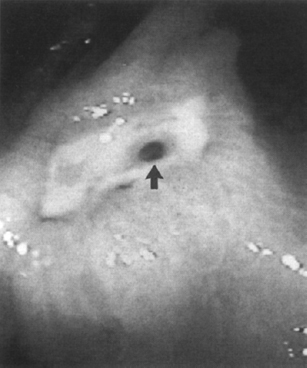
Figure 1-1 An atrophic vessel (arrow) in an ulcer crater appearing as a flat red spot. Therapy for Helicobacter pylori was prescribed. (See color plate 1–1.)
Therapeutic Endoscopy: Injection Techniques
Variceal Injection Sclerosis Injection sclerosis for the treatment of esophageal varices, first reported in 1939,82 was not popularized until the 1970s when fiberoptic flexible endoscopes became widely available. Sclerosing solutions are injected via a needle that is passed through the endoscope channel, allowing direct visualization of the index varix. It doesn’t seem to matter whether the injections are made directly into or next to the varices, as long as hemostasis is achieved.83,84 Commonly used sclerosants include the fatty acid derivatives morrhuate sodium and ethanolamine oleate, as well as synthetic oil compounds such as sodium tetradecyl sulfate and polydocanol, ethanol, and cyanoacrylate. These agents cause a necroinflammatory response resulting in thrombosis and fibrosis. Unwanted side effects include local ulcerations, fever, chest pain, bacteremia, pleural effusion, and, rarely, esophageal stricture.85 The concentrations and amounts injected per site and per sclerotherapy session vary considerably, and there is no consensus as to the optimal technique.86 Acute hemorrhage is controlled in 75 to 100% of patients with bleeding esophageal varices who receive sclerotherapy plus pharmacotherapy, in comparison with those who receive balloon tamponade with or without pharmacotherapy where control of hemorrhage is less successful (Fig. 1–5).87–90 Repeat treatments are continued until varices are completely sclerosed. Intervals are usually set at 1–2 weeks to allow sclerosis site ulcerations to heal, while ensuring compliance and limiting the at-risk time for rebleeding. Bleeding risk correlates with variceal size and stage of cirrhosis, with larger varices and Child C patients having predictably higher portal pressures, higher hepatic venous pressure gradients, and higher rates of variceal hemorrhage.91 Sclerotherapy is clearly superior to no treatment for the prevention of rebleeding,92–94 but it does not reduce portal pressure. The rebleeding risk between sclerotherapy sessions is 40 to 50%, compared with 70% or higher without any therapy. It is important to combine pharmacologic therapy with sclerotherapy to maximally reduce portal pressure medically while obliterating varices at risk for hemorrhage with sclerotherapy.32,94 Even after varices are endoscopically ablated, surveillance endoscopies should be performed, as varices may recur unless definitive lowering of portal pressure has occurred. Injection therapy for bleeding gastric varices is performed less often for many reasons. Although proven to be present in 75% of patients studied by portal vein catheterization,95 gastric varices are made visible by endoscopy in less than 10% of patients with portal hypertension (Fig. 1–6).96 Their bleeding incidence is highly variable, and bleeding is more difficult to control with medical therapy, tamponade, or injection therapy. This is probably related to their larger size and higher intravariceal pressure. Injecting larger volumes of sclerosant per varix has increased the successful control of active bleeeding.97 Several recent trials using the tissue “superglue” isobutyl-2-cyanoacrylate have shown 93 to 100% control of acute gastric variceal bleeding;98–100 isobutyl-2-cynnoacrylato immediately polymerizes upon contact with blood to form a tough adherent clot. Follow-up elective sclerotherapy for obliteration of gastric varices is unusual; transjugular intrahepatic portosystemic stent shunting (TIPSS), surgical shunting, or liver transplantation is most often recommended after gastric variceal hemorrhage, except in some cases of isolated gastric varices caused by splenic vein thrombosis wherein splenectomy is recommended.
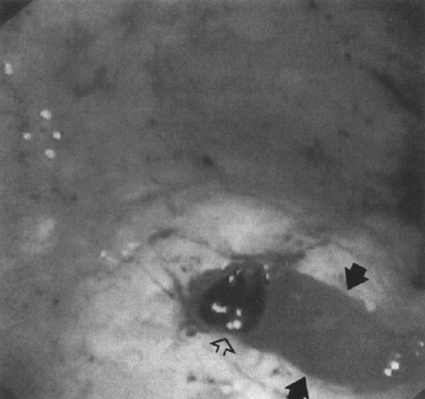
Figure 1–2 Active bleeding (arrows) from the base of a black clot (open arrow) obscuring an ulcer. Injection therapy was successful. (See color plate 1–2.)
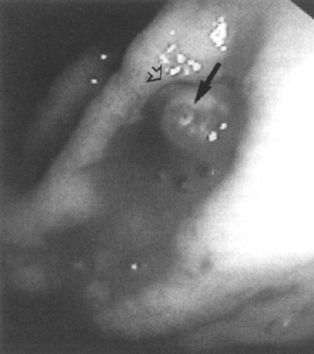
Figure 1–3 Visible vessel (arrow) with surrounding edematous folds (open arrow) from injection therapy. Rebleeding did not occur. (See color plate 1–3.)
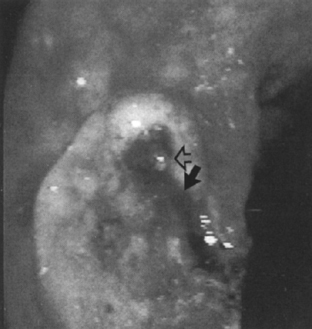
Figure 1–4 Minimal bleeding (arrow) from a pigmented protuberance (open arrow) in a large antral ulcer. Injection therapy halted further bleeding. (See color plate 1–4.)
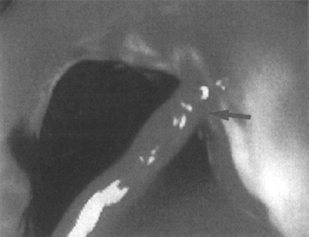
Figure 1–5 Active hemorrhage (arrow) from an esophageal varix seen only after rolling the patient to position the varix above the air-blood level. Sclerotherapy stopped the acute bleeding. (See color plate 1–5.)
Injection Therapy for Visible Vessels and Mucosal Tears Endoscopic injection therapy for bleeding peptic ulcers significantly reduces rebleeding, transfusion requirements, and need for urgent surgery compared with medical therapy alone.101 Injection techniques have been used for all types of vessels (arteries and veins) seen or suspected at nearby areas of visible oozing blood. Medicinal agents used to treat bleeding sites include absolute or diluted ethanol, normal saline, hypertonic saline, dilute epinephrine at 1:10,000 or 1:20,000, concentrated dextrose solutions, sodium tetradecyl sulfate, polydocanol (or other typical sclerosants), and combinations of these. Trials comparing efficacy using different agents show overall little difference, suggesting that tamponade alone may be the mechanism responsible for cessation of bleeding. Collectively, these agents are about 80% effective at initially controlling bleeding, with 20 to 30% recurrent bleeding usually occurring within 48 hours.81 When bleeding is massive or the patient presents with shock, a large bleeding artery is likely responsible.102 Several studies suggest that using a sclerosing agent for definitive obliteration of the artery is effective for the prevention of rebleeding.103–105 In dogs with experimentally induced ulcers, ethanol and polydocanol, but not epinephrine, caused definite arteriolar thrombosis.106 Ethanol and tetradecyl together resulted in the most effective mesenteric artery sclerosis but also caused significant transmural damage.107 Most endoscopists will safely inject 20 to 30 mL of dilute epinephrine circumferentially around vessels and then inject small amounts of a sclerosant directly into the vessel. Absolute ethanol should be used in 0.1- to 0.2-mL increments per injection site, not exceeding 1 to 2 mL total, to reduce the risk of transmural injury and the potential for perforation. Sclerosing agents may not be advisable for use in thin-walled areas, such as the duodenum or right colon. Advantages of injection therapy include low cost, relatively easy mastery of the technique, and low complication rates. Many endoscopists are combining injection therapy with hemostatic therapies, such as thermal coagulation. In a controlled trial, this combination was proven safe and effective,108 but its superiority over injection therapy or thermal coagulation alone has not been established. Randomized studies are needed to determine the optimal combinations associated with minimal risk and cost.
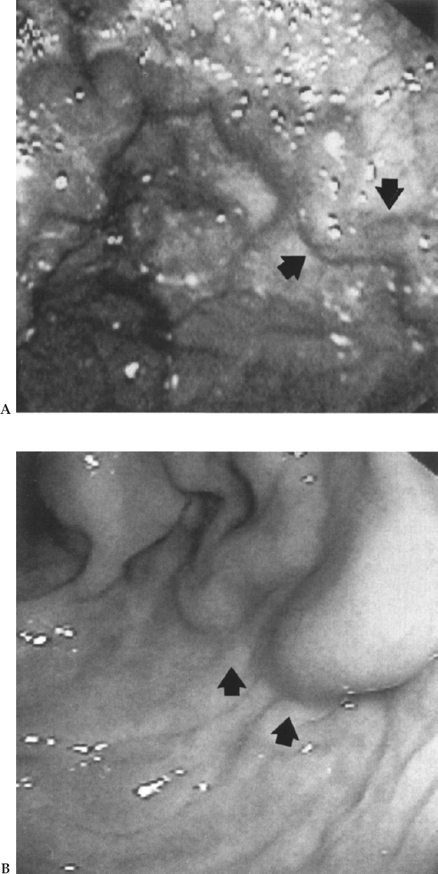
Figure 1–6 (A) Dark gastric varices (arrows) are easily recognized as veins compared with (B). (B) Light-colored gastric varices (arrows) may be overlooked as prominent rugae. (See color plate 1–6.)
Thermal Coagulation Techniques
Monopolar, Bipolar, and Heater Probe Devices Tissue cutting or coagulation results from heat generated when high-frequency current is passed through tissues that resist its flow. Monopolar circuits consist of current produced by a generator and flowing to an active electrode, through the patient, to a distant return electrode (grounding pad), and then back to the generator. In bipolar or multipolar circuits, the distance between the electrodes is minute, with current flowing through a very small amount of tissue. This allows for the use of lower pressure settings with less potential for complications. Therefore, most thermal coagulation probes in use today for endoscopic hemostasis are multipolar.109 Heater probes are computer-controlled thermal cautery devices set to deliver the desired amount of energy to a tip coated with a nonstick surface.110 The resulting tissue effect is essentially the same as with multipolar probes. Several randomized controlled trials have shown that thermal coagulation of bleeding or visible vessels is efficacious at controlling hemorrhage and decreasing re-bleeding.111–113 Depth of coagulation with the larger 3.2-mm probes varies with watt setting, tissue contact time, and applied pressure.114 High-power settings theoretically cut off the current when the tissue temperature reaches 100° C, as water vaporizes and dehydrated tissue conducts heat poorly115 Presence of desiccating tissue may also result in a larger hole in the vessel wall and exacerbation of bleeding. The optimal technique is to coapt opposing sides of the vessel wall by pushing firmly with the larger 3.2-mm probes and then slowly heat using low-power settings (15 to 25 W) with longer (10 seconds) contact times.114 Randomized trials comparing multipolar coagulation to laser, injection therapy, and heater probe have shown equivalent efficacy for hemostasis of bleeding peptic ulcers.116–119 Heater probes and multipolar thermal coagulation techniques have also been used for treatment of bleeding due to Mallory-Weiss tears, Dieulafoy’s lesion (Fig. 1-7), vascular angiomata, postsphincterotomy and postpolypectomy hemorrhage, watermelon stomach, and postirradiation-induced mucosal hemorrhage.112,120–125 Although low-power settings are optimal for these applications as well, care must be taken in thin-walled areas to tailor the pressure and tissue contact time to minimize the potential for perforation. A meta-analysis of 25 randomized controlled trials comparing the efficacy of thermocoagulation or injection therapy with that of nonendoscopic management for bleeding peptic ulcers revealed significantly reduced rebleeding, decreased need for surgery, and 30% reduction in mortality in patients treated with endoscopic therapy.126
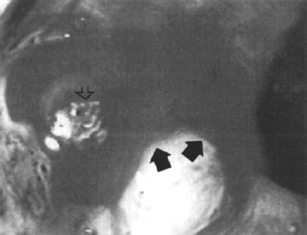
Figure 1–7 Blood (arrows) oozing from a Dieulafoy’s lesion (open arrow). Thermal coagulation was successful. (See color plate 1–7.)
Laser Endoscopic injection therapy and heater probe or multipolar thermal coagulation techniques are used most often for the management of acute upper GI bleeding because they are relatively inexpensive, effective, and portable. Laser therapy is more expensive and more difficult to master but very effective for all types of bleeding lesions. By 1973, it became feasible to pass both argon and neodymium:yttrium-aluminum-garnet (Nd:-YAG) laser energy through fiberoptic gastroscopes.127,128 Laser light is aimed at bleeding sites, with a plastic catheter waveguide placed through the endoscope channel. Laser energy is selectively absorbed by the red color of hemoglobin, giving this method a theoretical advantage in controlling hemorrhage. Photons of light that are absorded or scattered at the tissue surface produce heat. The amount of heat produced depends on the laser type and tissue exposure time. Tissue absorbs light of different wavelengths at various degrees; therefore, the argon, Nd:YAG, and CO2 lasers produce tissue coagulation to different depths. Because of its capacity to coagulate to a depth of 5 mm as well as produce a wider (circumferential) tissue effect from immediate scatter, the Nd:YAG laser is employed most often for gastrointestinal hemostasis. For optimal use, the recommendation is to use short pulses (0.5 to 1 second) of relatively high energy (60 to 90 J) in a tight circle immediately surrounding the bleeding site and aimed directly at the point of last bleeding.129 By 1990, the largest uncontrolled study of the use of Nd:YAG laser in 1058 patients with bleeding peptic ulcers showed that hemostasis was achieved in 94%, with a complication rate from perforation of less than 2%.130 Multiple randomized controlled trials comparing Nd:YAG laser with other forms of endoscopic hemostasis for peptic ulcer reveal a slight benefit of laser to other methods.131–133 Meta-analysis of trials of laser photocoagulation versus control for bleeding ulcers favored laser for significantly reducing rebleeding, controlling acute bleeding, reducing the need for surgery, and reducing mortality.134 Laser therapy has also been used in many patients to control acute and chronic bleeding from angiomata, telangiectasis in Osler-Weber-Rendu disease, and watermelon stomach.135–138
Argon Plasma Coagulation Argon plasma coagulation is a noncontact thermal technique that combines the technologies of monopolar electrocoagulation and laser photocoagulation. It is portable, easy to use, and less expensive than the Nd:YAG laser. Argon gas flows through a catheter as a spray on the target tissue surface, with transmission of the high-frequency electrical current to the gas by a thin electric wire at the tip of the catheter. This allows for rapid thermal treatment of a large surface area, with a maximum depth of coagulation at 3 to 4 mm.139 Trials with this therapy in patients with a variety of GI bleeding lesions revealed excellent hemostasis and safety.140,141
Mechanical Techniques
Endoscopic Clips Metallic clips for endoscopic hemostasis were first introduced in Japan in 1975.142 These devices are most useful when the exact site of bleeding is visible. As there is essentially no damage to surrounding tissues, endoclip devices are ideal for thin-walled segments of the GI tract, such as a protruding artery in the base of a deep duodenal ulcer or bleeding from postpolypectomy sites. Studies comparing the use of hemoclips with other hemostatic therapies showed no significant difference in efficacy.143,144
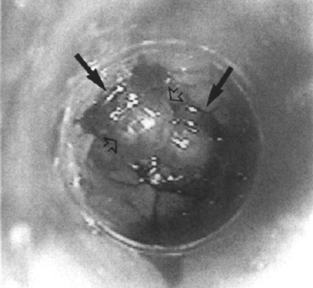
Figure 1–8 Endoscopic view after deploying multiple rubber bands (arrows) on esophageal varices (open arrows). (See color plate 1–8.)
Band Ligation Band ligation for the treatment of esophageal varices was first introduced in 1988.145 Today band ligation has exceeded injection sclerosis as the primary method of elective endoscopic treatment for esophageal varices (Fig. 1–8). Compared with injection sclerosis, band ligation is as effective but associated with fewer complications of infection, esophageal stricture, and site ulceration.146–149 Band ligation has also been used to control bleeding attributed to vascular angiomata, Dieulafoy’s lesion, Mallory-Weiss tears, and peptic ulcers.150–152
Endoscopic Tattooing Unusual lesions, such as Dieulafoy’s lesion or other lesions that are difficult to access and require subsequent endoscopic or surgical therapy, can be tattooed at the initial endoscopy to aid in their relocation. India ink diluted with 1:10 or 1:100 saline can be autoclaved or passed through a microfilter 153 prior to use. It is injected in increments of 0.1 to 0.5 mL at or circumferentially around lesions and later can be seen on either the gut mucosal or the serosal surface.154–155 Ink tattoos remain in the tissues for years and are considered permanent.156
Radiologic Diagnosis and Management
Nuclear Scintigraphy
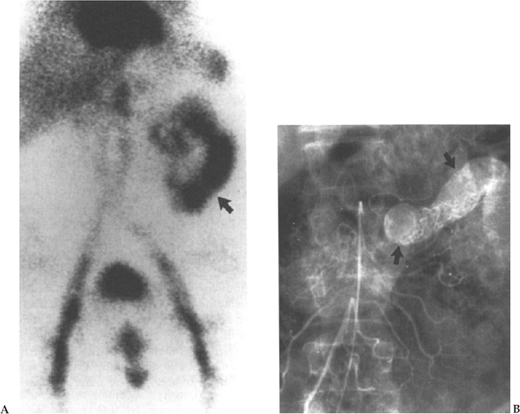
Nuclear GI scintigraphy may be helpful in patients with obscure GI bleeding. Most often, patients have already had one or more endoscopic evaluations with negative findings. A typical scenario is an elderly person without abdominal pain who intermittently experiences melena or passage of maroon-colored stools. Lesions may be anywhere from the nasal mucosa to the right colon. Vascular angiomata are a common source of bleeding in this setting,10,157,158 whereas duodenal or jejunal diverticuli, neoplasias, hemobilia, or other lesions are less likely. A small bowel enteroscopic examination would ideally be used for diagnosis and coagulation therapy. However, if such an examination is unavailable or nondiagnostic, nuclear scintigraphy may be beneficial (Fig. 1–9A, 9B).
Labeling a patient’s red blood cells with technetium pertechnetate allows for immediate and delayed scanning; the tracer remains sufficiently detectable for about 24 hours. Bleeding rates as low as 0.1 to 0.5 mL/min (one half to two units packed red blood cells/24 h) can be detected.159 Technetium 99–labeled sulfur colloid injected intravenously is followed by immediate scanning and hence is limited to patients with active bleeding. In the clinical setting, nuclear scintigraphy can identify bleeding with 40 to 65% sensitivity and 25% specificity. Low specificity is explained by dispersion of the tracer once it has extravasated into the gut lumen.
Angiography
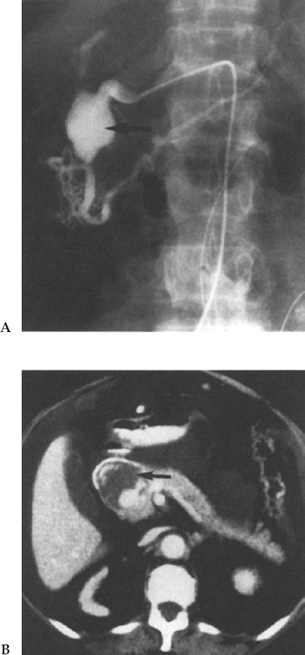
Angiography is an excellent adjunct to both diagnosis and treatment of brisk GI bleeding. It is usually employed when standard diagnostic endoscopies are non-diagnostic or therapeutically unsuccessful (Fig. 1–10). Although most helpful in cases of voluminous lower GI bleeding, where colonoscopic visualization of the mucosa is markedly impaired, many rapidly bleeding upper GI lesions escaping endoscopic control can be managed with angiography. Selective celiac and superior mesenteric artery catheterization outlines the arterial supply of the distal duodenum and proximal jejunum. Superselection of smaller arteries with microcatheters and steerable wires allows for localization of very distal bleeding arterial branches. The formerly popular agent vasopressin with its potent arteriolar smooth-muscle constricting effect is used less frequently now due to the unwanted systemic side effects, which include reduced renal and mesenteric blood flow, coronary ischemia, myocardial infarction, and arrhythmias.160
Angiographic evaluation can be tailored for a specific site if a nuclear medicine study indicates a distinct focus of bleeding. This, however, does not exclude the possibility of a second GI bleed, although that is statistically less likely. If no distinct focus of bleeding is predetermined from either nuclear medicine or endoscopic studies, then a full mesenteric evaluation is necessary.
Bleeding is classified as being either upper or lower GI in origin. Upper GI bleeding evaluation begins with a celiac arteriogram. The left gastric branch of the celiac artery should be of particular interest given its supply to the stomach, as should be the gastric duodenal artery, which is commonly involved in duodenal ulcerations. If no source of bleeding is identified, then the next vessel for study should be the superior mesenteric artery (SMA), followed by the inferior mesenteric artery (IMA); these are the primary vessels involved in lower GI bleeding. It has been recommended that, prior to IMA angiography, limited aortography be performed because atherosclerotic disease at this level may make catheterization of this vessel risky.
When evaluating the mesenteric vessels, prolonged imaging is of paramount importance for detecting any staining or blush, which may represent a hemorrhage. Recognizing a bleed can be difficult with subtraction images, secondary to bowel motion. Glucagon can be used to help decrease peristalsis prior to filming. Also, the images can be viewed in the nonsubtracted mode. An alternative to conventional contrast, CO2 angiography is being discussed as an approach to GI bleeding. Being less viscous then iodinated contrast, CO2 easily traverses hemorrhagic vessels and expands as it becomes extravascular, allowing easier visualization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree