Gastric Cancer: Epidemiology, Screening, Surveillance, and Prevention
Rene Lambert
Donald M. Parkin
Incidence Survival and Mortality from Gastric Cancer
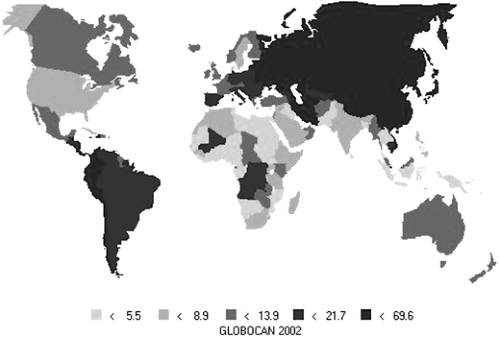
Despite a declining incidence, stomach cancer is still one of the most common causes of cancer, and the second most common cause of death from cancer worldwide (1). The areas of highest incidence and mortality are in eastern Asia (Japan, Korea, China), in the Andes in South America, and in eastern Europe (2), as shown in Table 19.1 and Fig. 19.1. Incidence in men is about twice that of women in both high- and low-risk countries, although close inspection of age-specific incidence data (3) shows that rates in women exceed those of men in the youngest age groups (younger than age 40).
In Japan, stomach cancer is still the most common type of cancer overall (4,5,6). In an estimate based on data from 11 population-based registries, stomach cancer accounted for 23% of cancer cases in males in 1999 and 15% in females (6). Although the lifetime risk of developing gastric cancer has been decreasing in Japan, the increasing proportion of elderly persons means that the actual toll, in terms of new cases per year, is still increasing despite a declining age-specific and age-standardized incidence.
In the Western world, the declining incidence of cancer in the distal stomach contrasts with an increase of cancer at the esophagogastric junction (including the cardia) (7). Although there is some inaccuracy in attributing the site of origin of cancers at the esophagogastric junction to stomach or oesophagus (8), such misclassification cannot account for the large changes observed.
In its early stage, stomach cancer is either asymptomatic or presents with nonspecific symptoms that suggest dyspepsia. In the Western world, most cases are detected at an advanced stage, with invasion of the muscularis propria or the serosa, and with a correspondingly poor prognosis. In the United States and Europe, the 5-year relative survival rate is around the 20% level (9,10), as shown in Table 19.2, despite a weak trend to improvement (Fig. 19.2). In the United States, an analysis of 57,407 cases in the National Cancer Data Base (11) showed that most cases in the 1987–1988 cohort were still detected at an advanced stage, with as many as 39.9% in stage IV. In the 1992–1993 cohort, at each stage of the disease, the number of cases that received no treatment is surprisingly high (28.7% on average), and there were many nontreated patients even with early stage disease (38.5% in stage IA, 26.1% in stage IB). Survival in developing countries is still lower (<15%) than in developed countries (12)
In Japan, survival is considerably better (>40%) than in the Western world, even in population-based series (13,14), as shown in Fig. 19.2. The trend to increased survival during the last 25 years also concerns the cases detected outside the official screening programs (Table 19.3). This difference suggests that the national policy of prevention is beneficial. Survival is influenced by the distribution of cases by stage and by survival within stage:
The localized (curable) stage is much more frequent in Japan than in the West.
The regional stage has better survival in Japan than in the West. The respective 5-year survival rates (both genders) in the first half of the 1980s were 32.0% in the Osaka Cancer Registry (5) and only 15.0% in the Surveillance, Epidemiology, and End Results (SEER) registries (white) of the United States (9).
The distant stage (metastases) has the same poor prognosis in the East as in the West.
The declining incidence in Japan shows that the “unplanned prevention” (15) observed in other countries is also occurring in Japan, presumably as a result of changing lifestyle. Nevertheless, secondary prevention has been a priority in Japan since 1963; it aims at early diagnosis of gastric cancer in asymptomatic persons. Time trends in incidence and mortality may help assess the role of screening. The sustained decrease of the mortality-to-incidence ratio after 1970 in Japan strongly supports a beneficial effect. The trend is shown in Table 19.4 with data from the Osaka Cancer Registry (16). However, favorable survival and mortality to incidence ratios may also be due to other factors:
Incidence may be somewhat inflated by the detection of cancers (by screening) that would not otherwise have been diagnosed within the life span of some individuals (overdiagnosis).
A different classification of intramucosal neoplasia without invasion increases the numbers of favorable cases, including as “cancer” many of the so-called high-grade dysplasia lesions in the Western world.
Stage migration (17) is linked to lymphadenectomy D2 in the classification of cancer at the regional stage, so tumors previously classified N1 are classified N2.
Table 19.1 ASR incidence (for 100,000) of stomach cancer (1993–1997) in some regions with a high risk as compared to the low incidence rate in the United States | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
Etiology in Relation to Prevention
Most gastric cancers occur sporadically and are related to environmental factors, linked to diet and infection. Atrophic gastritis may be an important intermediary because it results in the increased intraluminal pH, which may promote the genesis of carcinogens in the gastric lumen. A study in cancer registries of China (18) illustrates the role of lifestyle: Mortality from gastric cancer (ASR/100,000) has been compared in the urban and rural populations in two periods (1973–1975 and 1990–1992). In urban areas, the rate decreased by 23.8%, whereas in rural areas it increased by 25.8%.
Table 19.2 Relative 5-yr survival rate (both genders) for stomach cancer in Japan, America, and Europe | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
Role of Genetic Susceptibility
Family relatives of gastric cancer patients have a higher risk of cancer, with a high prevalence of Helicobacter pylori infection and intestinal metaplasia in the stomach (19). However, only a small proportion of gastric cancers are due to genetic predisposition, as characterized by familial clustering and a dominant inheritance pattern. The rare syndrome of hereditary diffuse gastric cancer has been described in New Zealand and is attributed to a germline mutation in a gene encoding the cell adhesion protein E-cadherin (20,21). This cancer is a diffuse, poorly differentiated infiltrative adenocarcinoma. In contrast, gastric cancer of the intestinal differentiated type may occur in hereditary nonpolyposis colon cancer (22) or in patients with gastrointestinal polyposis, including familial
adenomatous polyposis and Peutz-Jeghers syndrome. With respect to the infectious factor, genetic polymorphisms may play a role, both for H. pylori and for the human host. A small increase in the risk of gastric cancer (23) is linked to the blood group A phenotype and explained by the higher risk of atrophic gastritis with the adhesion of H. pylori to the Lewisb blood group antigen.
adenomatous polyposis and Peutz-Jeghers syndrome. With respect to the infectious factor, genetic polymorphisms may play a role, both for H. pylori and for the human host. A small increase in the risk of gastric cancer (23) is linked to the blood group A phenotype and explained by the higher risk of atrophic gastritis with the adhesion of H. pylori to the Lewisb blood group antigen.
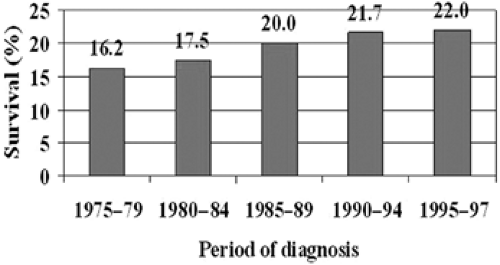 FIGURE 19.2. Five-year relative survival of stomach cancer in the United States. Source: From ref. 9. |
Role of Dietary Factors
A high intake of mucosal irritants such as salt and nitrates causes chronic inflammation and superficial gastritis, which can develop into atrophic gastritis (24,25). The fact that dietary salt also influences the more advanced pathological changes of the gastric mucosa was demonstrated in a cohort study in Japan (26), where during a 6-year follow-up of 5,373 persons, 69 developed gastric cancer. The risk of gastric cancer increased 2.2-fold in subjects with atrophic gastritis at enrollment; in the presence of atrophic gastritis, the risk was 1.8-fold by the consumption of spicy food and decreased 0.6-fold by a reduction in intake of salty food.
Reduced acid secretion in the presence of atrophy increases gastric pH, thus favoring the growth of anaerobic bacteria, which reduces nitrate to nitrite and can eventually form N-nitroso mutagens. In contrast, a high intake of fruit and vegetables is associated with a reduced risk of gastric cancer (27). Antioxidants such as beta-carotene, alpha-tocopherol (vitamin E), and ascorbic acid (vitamin C) prevent the formation of mutagens and carcinogens in the stomach. The exact role of these vitamins in the etiology of gastric cancer is not known, and it may be that low ascorbic acid in gastric juice is simply a marker of the extension of inflammation and infection.
Table 19.3 Time trends in detection and relative 5-yr survival rate of stomach cancer in Japan: the Osaka Cancer Registry, 1975–1989, in men | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
Table 19.4 Time trend of age-standardized (world standard) Incidence and Mortality per 100,000 for stomach cancer in Japan | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||
Role of Tobacco and Alcohol
In its recent review, the International Agency for Research on Cancer (IARC) (28) concluded that there was a consistent and causal association of cancer of the stomach with cigarette smoking in both men and women. Confounding by other factors (alcohol, H. pylori infection, and dietary factors) could be reasonably ruled out. Risk increases with duration of smoking and number of cigarettes smoked, and decreases with increasing duration of successful quitting.
Role of Helicobacter pylori
In 1994, H. pylori was classified by IARC (31) as being carcinogenic for humans, with sufficient evidence for a causal association with both carcinomas of the stomach and gastric lymphoma. At that time, much of the evidence came from retrospective case-control studies. However, these studies are limited in that the presence of H. pylori infection is evaluated after the development of cancer. H. pylori tends to disappear as intestinal metaplasia and atrophy develop, so prevalence of infection may be seriously underestimated in cases, even if anti–H. pylori antibody is used as an indicator of infection. Prospective studies
give a more accurate assessment. Several case-control studies nested within cohorts have now been published in which infection is evaluated in cases and controls before the onset of disease. The results of these studies have been the subject of several meta-analyses (32,33,34,35). In the review by the Helicobacter and Cancer Collaborative Group (35), 12 prospective studies were included, with a total of 1,228 gastric cancer cases and 3,406 controls. Overall, the odds ratio (OR) for the association between H. pylori infection and the subsequent development of gastric cancer was 2.36 (95% confidence interval [CI], 1.98–2.81). Analyzing cancers of the gastric cardia and noncardia separately, the authors found no increase in risk for cardia cancers (OR = 0.99), while the overall risk for noncardia cancers was 2.97 (95% CI, 2.34–3.77). The risk varied with the interval between sample collection and cancer diagnosis (as might be expected, if infection is progressively lost as gastric atrophy develops). The increase in risk was 5.9-fold (95% CI, 3.4–10.3) for H. pylori positivity 10 years or more prior to diagnosis. The associations were not related to histologic type of gastric cancer (intestinal vs. diffuse) or gender.
give a more accurate assessment. Several case-control studies nested within cohorts have now been published in which infection is evaluated in cases and controls before the onset of disease. The results of these studies have been the subject of several meta-analyses (32,33,34,35). In the review by the Helicobacter and Cancer Collaborative Group (35), 12 prospective studies were included, with a total of 1,228 gastric cancer cases and 3,406 controls. Overall, the odds ratio (OR) for the association between H. pylori infection and the subsequent development of gastric cancer was 2.36 (95% confidence interval [CI], 1.98–2.81). Analyzing cancers of the gastric cardia and noncardia separately, the authors found no increase in risk for cardia cancers (OR = 0.99), while the overall risk for noncardia cancers was 2.97 (95% CI, 2.34–3.77). The risk varied with the interval between sample collection and cancer diagnosis (as might be expected, if infection is progressively lost as gastric atrophy develops). The increase in risk was 5.9-fold (95% CI, 3.4–10.3) for H. pylori positivity 10 years or more prior to diagnosis. The associations were not related to histologic type of gastric cancer (intestinal vs. diffuse) or gender.
In the human host, H. pylori infection can be detected by anti–H. pylori antibodies. It is an exceedingly common infection with an estimated overall prevalence of infection in middle-age adults of 74% in developing countries and 58% in developed countries (36). The prevalence of infection increases rapidly with age, is associated with childhood living conditions (37), and may persist during a long period. In adults undergoing a periodic health check, the rate of seroconversion to positive and of seroreversion to negative over 9 years was estimated as 6.3% and 7.1%, respectively (38).
Prevalence of infection correlates reasonably well with the risk of gastric cancer in the same population (31) but is not perfect. The “African enigma” (39) refers to the supposed low incidence of gastric cancer in Africa, where there is high prevalence of H. pylori infection. However, the incidence of gastric cancer in Africa (particularly central Africa) is not especially low (40), and even if it were, there is no reason to suppose that the risk related to H. pylori could not be modified by other (protective) factors. An evidence-based review (41) of 40 prospective endoscopic studies from 17 African countries (20,531 persons) did not suggest dissociation between the prevalence of H. pylori infection and H. pylori–related diseases: H. pylori–related clinical outcomes included duodenal ulcers in 21.1%, gastric ulcers in 3.4%, and gastric cancers in 2.4%.
Studies using serum banks suggest that there has been a decline in prevalence of infection with H. pylori between successive generations (42,43,44,45). Banatvala et al. (44), for example, using 631 sera collected in 1969, 1979, and 1989, observed that the odds of being H. pylori seropositive decrease by 26% per decade. An early age of infection favors the development of atrophic gastritis and increases the risk of stomach cancer. From 1973 to 1994 in Finland (45), the proportion of subjects infected by H. pylori strains has declined. However, the prevalence of cag-A (+) strains among those younger than 45 years declined more than for cag-A (–) strains. In Japan (46), middle-age persons have a pattern of infection similar to that in developing countries, whereas young generations have a much lower rate of infection. Time trends in the incidence of gastric cancer in Japan were analyzed from 1976 to 1996 and compared to changes in the prevalence of H. pylori infection (47), which declined in both genders between 1989 and 1998, with young age groups experiencing more change. In addition, a marked decline in gastric cancer incidence occurred in the young population ages 20 to 39 years. An estimation of the numbers of stomach cancer attributable to H. pylori infection in the world for 2002 (29) is shown in Table 19.5.
Table 19.5 Estimated numbers of gastric cancer cases (both genders) in 2002 caused by H. PYLORI, in developed and developing countries | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
The recent determination of the complete DNA sequence of the H. pylori genome (48) has shown specific islands common to various strains, but all strains of H. pylori may not play the same role in the development of gastric cancer. In humans, subjects infected with cag-A–positive strains have a higher prevalence of atrophic gastritis and higher levels of IgG anti–H. pylori antibodies than those with cag-A–negative infections (49,50,51). H. pylori can also produce a vacuolating cytotoxin named vac-A. In Colombia, the relative frequency of cag-A and vac-A s1 and m1 phenotypes was higher in gastric biopsies from high-risk areas for gastric cancer (49) than in low-risk areas.
The etiologic role of H. pylori in gastric carcinogenesis is supported by animal experiments showing that inoculation of cag-A– and vac-A–positive strains was able to induce intestinal metaplasia and gastric carcinoma in Mongolian gerbils (52). Research is ongoing to determine whether strain-specific genes could be associated with gastric carcinogenesis (53).
The carcinogenic effect of H. pylori is indirect and linked to the increased proliferation of the gastric epithelium occurring in the H. Pylori–infected stomach. This effect depends on the ability of H. pylori to produce ammonia via its potent urease action on intragastric urea. Cell replication potentiates the action of carcinogens targeting DNA (54,55,56,57,58). The bacterial organisms are hosted in the mucus layer overlying the normal gastric epithelium but not in areas overlying intestinal metaplasia where neoplasia originates; this suggests that soluble carcinogens present in the gastric lumen play a role. H. pylori infection causes oxidative stress in the gastric mucosa; inducible nitric oxide synthetase (iNOS) is expressed in inflammatory cells and located in the lamina propria at the early stage of gastritis (58). Nitric oxide (NO) is active on the renewal sector of epithelial
cells in the deep foveolar epithelium and in lymphoid follicles. In the precancerous mucosa, iNOS migrates in the superficial foveolar epithelium, where protein and DNA damage can be produced. At this stage, bacterial eradication diminishes the markers without preventing new DNA damage. The higher intragastric pH following atrophic gastritis provoked by H. pylori may rather modify external or endogenous carcinogens. Nitrosated compounds are recognized as gastric carcinogens in the experimental setting. Transformation of nitrite (NO2) to NO produces the reactive dinitrogen trioxide (N2O3) that forms nitrosothiols and nitrosamines. This chain reaction toward nitrosation is inhibited in the presence of antioxidants, explaining the preventive action of ascorbic acid; however H. pylori also interferes with antioxidant functions by decreasing intragastric ascorbic acid concentrations.
cells in the deep foveolar epithelium and in lymphoid follicles. In the precancerous mucosa, iNOS migrates in the superficial foveolar epithelium, where protein and DNA damage can be produced. At this stage, bacterial eradication diminishes the markers without preventing new DNA damage. The higher intragastric pH following atrophic gastritis provoked by H. pylori may rather modify external or endogenous carcinogens. Nitrosated compounds are recognized as gastric carcinogens in the experimental setting. Transformation of nitrite (NO2) to NO produces the reactive dinitrogen trioxide (N2O3) that forms nitrosothiols and nitrosamines. This chain reaction toward nitrosation is inhibited in the presence of antioxidants, explaining the preventive action of ascorbic acid; however H. pylori also interferes with antioxidant functions by decreasing intragastric ascorbic acid concentrations.
Progression of the Disease
The pathological changes that precede cancer are chronic atrophic gastritis, metaplasia of the intestinal type, and dysplasia. Dysplasia is intraepithelial neoplasia without invasion of the stroma; it arises in either the normal gastric epithelium or in intestinal metaplasia. In gastric oncogenesis, dysplasia lies between atrophic metaplastic lesions and invasive cancer. Progression of the disease was studied in a follow-up study of 1,400 asymptomatic subjects in an area of high gastric cancer risk in Colombia (59,60); endoscopy and biopsy were performed twice, at an average interval of 5 years, and showed an overall progression to more advanced lesions. Atrophic gastritis preceded gastric cancer in a Japanese prospective study (26), which reported a relative risk of 2.3 of developing cancer for those affected by severe atrophy. The less severe types of lesions—both nonatrophic and mild atrophic gastritis—have also been shown to occur more often in cases of gastric cancer than in control subjects. A case-control study (61) in Finland, including 243 cases with gastric cancer and 1,408 controls for which gastric histology was available found a relative risk of gastric cancer in patients with nonatrophic antral gastritis of 1, 8, and 2.5 in patients with nonatrophic pangastritis. The same group (62) observed a declining prevalence of gastritis in Finland from 1977 to 1992 in young age groups (similar to the decrease in incidence of gastric cancer). The diagnosis of gastric epithelial dysplasia includes (a) the distinction between true dysplasia and the reactive changes associated with inflammation, and (b) the distinction between dysplasia and truly invasive cancer.
In a meta-analysis of the literature (63), 80% of cases of high-grade dysplasia will progress to cancer after 6 months, whereas the rate of transformation of low-grade dysplasia is 10% in 1.5 years. The speed of progression from early to advanced cancer is not constant, and it is possible that some of the tumors detected might not have manifested themselves later. The potential role of this bias has been examined in Japan (64) in a series of 43 early gastric cancer cases who received no treatment for at least 6 months and had some follow-up (diagnostic tests, late operation). Advanced gastric cancer occurred in 27 subjects (63%); the median duration of early gastric cancer after diagnosis was 37 months. This suggests that most such early cancers diagnosed through screening will progress to more advanced symptomatic disease.
In Western countries, the term “adenoma” is applied when the mucosal proliferation produces a macroscopic protruding lesion. Gastric adenomas are less common than hyperplastic polyps; they account for approximately 10% of gastric polyps. Malignant transformation of adenomas is rare in lesions measuring <2 cm but frequent (40%) in lesions >2 cm. Flat adenomas may have a greater tendency to develop into carcinomas, even when small.
In Japan, intraepithelial neoplasia with severe dysplastic changes and no invasion of the lamina propria is also called intramucosal carcinoma. In Western countries, a diagnosis of intramucosal carcinoma is made only if there is demonstration of malignant cells in the lamina propria. The East/West divergence, confirmed by multidisciplinary teams (65), disappears when there is tumor invasion across the muscularis mucosae. The inclusion of these noninvasive “intramucosal” carcinomas in Japanese series of gastric cancers accounts, at least in part, for the markedly better prognosis of gastric cancer in Japan.
A significant distinction in the morphology of gastric cancer is between the differentiated intestinal and the poorly differentiated diffuse types (66). The first is linked to chronic atrophic gastritis and intestinal metaplasia, and is responsible for most geographic variations in risk (67). In Japan, the decline in incidence of gastric cancer is largely attributed to decline in the intestinal type of gastric carcinoma (68). Similar, although less clear, changes have been seen in U.S. data (69). Molecular genetics is becoming increasingly important, and several loci have been identified, indicating possible tumor-suppressor genes. As yet, none of these tests is currently used in the screening of the early stages of gastric cancer.
Direct Diagnosis of Cancer
Gastroscopy
Gastroscopy has not been used as the primary test in mass screening. Leaving aside issues of cost, it does not satisfy several other criteria for a screening test, one of which relates to complete safety. There is a very small (but not nil) risk of severe complications, either respiratory or digestive (hemorrhage or perforation). In a retrospective study of 211,410 procedures (70), complications and mortality occurred in 0.13% and 0.04%, respectively, and in a prospective study from a single institution; the rate of complications was as high as 1.9% (70). Although not used as a screening test, gastroscopy is considered as a “gold standard” against which to evaluate sensitivity of other procedures in the detection of gastric malignancy and permit biopsies. Gastroscopy also proved efficacy in the morphologic distribution of superficial cancer in the subtypes of a type 0 occurring in superficial cancer (Table 19.6) (71).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree