Study (location)
Year
GBCA exposure
Cases of GIF/GBCA exposed patients with renal disease
Notes
Marckmann (Denmark)
2006
Gadodiamide
13/370 (3.5 %)
Retrospective chart review: strong association of gadodiamide exposure with GIF (OR 32.5)
Broome (Loma Linda, CA)
2007
Gadodiamide
N/A
Retrospective chart review: 12/168 HD patients (7.1 %) had GIF, and all had been exposed to gadodiamide (OR 22.3)
Collidge (Scotland)
2007
Gadodiamide
13/421 (3.1 %)
Retrospective chart review: significant association between gadodiamide exposure and GIF (rate ratio 6.35). Dose response observed
Deo (Bridgeport, CT)
2007
Gadodiamide
3/87 (3.4 %)
Retrospective chart review
Lauenstein (Atlanta, GA)
2007
Gadodiamide
8/312 (2.6 %)
Retrospective chart review
Othersen (Charleston, SC)
2007
Gadodiamide
4/261 (1.5 %)
Retrospective chart review
Todd (Boston, MA)
2007
Gadopentetate
16/54 (29.6 %)
Cross-sectional cohort study: strong association between gadopentetate exposure and GIF (OR 14.7). Increased mortality among GIF patients vs. controls (HR 4.1)
Prince (New York, NY)
2008
Gadodiamide
Gadopentetate
Gadobenate
Gadoteridol
15/786 (1.9 %)
Retrospective chart review: 15 GIF cases among 786 patients with eGFR <30 or AKI. Of these, 14/653 (2.1 %) patients exposed to gadodiamide developed GIF
Reilly (Dallas, TX)
2008
Gadoteridol
0/141 (0 %)
Retrospective chart review: 141 HD patients who had 198 exposures to gadoteridol. No GIF cases reported
Rydahl (Denmark)
2008
Gadodiamide
18/102 (17.6 %)
Retrospective chart review
Wertman (Chapel Hill, NC)
2008
Gadodiamide
Gadopentetate
N/A
Retrospective chart review: four academic centers. GIF rates greater at institutions that used gadodiamide, (32/82,260) compared to gadopentetate (4/135,347), with denominator being all patients regardless of renal function
Abujudeh (Boston, MA)
2009
Gadobenate
0/250 (0 %)
Retrospective chart review: no incident cases of GIF among patients (mostly stage 3 CKD)
Altun (Chapel Hill, NC)
2009
Gadodiamide
Gadopentetate
Gadobenate
37/1237 (3.0 %)
Retrospective chart review: all cases of GIF were associated with gadodiamide. No cases of GIF in 549 patients after switch to gadopentetate or gadobenate for at-risk patients
Chen (Taiwan)
2009
Gadodiamide
1/127 (0.8 %)
Retrospective chart review
Chrysochou (the United Kingdom)
2009
Gadodiamide
Gadopentetate
Gadofosveset
0/1659
Retrospective chart review: 1659 patients had stage 3, 4, or 5 CKD. No cases of GIF were reported, but, of the patients with stage 4 or 5 CKD, only 14 received gadodiamide, and only 246 received gadopentetate dimeglumine. None of the other patients received a “high-risk” GBCA
Heinz-Peer (Austria)
2009
Gadodiamide
Gadopentetate
Gadoterate
Gadobutrol
Gadoteridol
Gadobenate
Gadoxetate
6/367 (1.6 %)
Retrospective chart review
Hope (N. California)
2009
Gadopentetate
1/530 (0.2 %)
Retrospective chart review
Lee (Rochester, MN)
2009
Gadodiamide
Gadopentetate
Gadobenate
Gadoteridol
Gadoxetate
8/827 (1 %)
Retrospective chart review
Perez-Rodriguez (Baltimore, MD)
2009
Gadodiamide
Gadopentetate
N/A
Lemy (Belgium)
2010
Gadodiamide
5/33 (15.2 %)
Retrospective chart review: 705 patients who had undergone renal transplantation, only 33 of whom had been exposed to a GBCA over a 7-year period
Martin (Atlanta, GA)
2010
Gadodiamide
Gadobenate
8/1096 (0.7 %)
Retrospective chart review: 8 of 312 (2.6 %) HD patients developed GIF after gadodiamide exposure (dose response). None of 784 HD patients developed GIF after the institution switched to the more thermodynamically stable GBCA, gadobenate
Chow (Los Angeles, CA)
2011
Gadodiamide
Gadopentetate
1/357 (0.3 %)
Retrospective chart review: 2142 patients who had undergone liver transplantation. Of these, 200 had stage 3 CKD, 60 had stage 4 CKD, and 97 had stage 5 CKD. One case of GIF occurred in a patient with stage 5 CKD who had received gadodiamide
Kendrick-Jones (New Zealand)
2011
Gadodiamide
Gadopentetate
Gadobenate
Gadobutrol
5/522 (1.0 %)
Retrospective chart review: 522 chronic HD patients who had a total of 748 GBCA exposures. GIF was observed following 5 (1.3 %) of 392 gadodiamide-enhanced MRI studies and none of 356 studies using an alternative GBCA
Wang (Boston, MA)
2011
Gadopentetate
Gadobenate
N/A
Retrospective chart review: Restrictions placed on GBCA use in patients with renal disease (2008). Before, there were 1287 GBCA exposures among patients with GFR < 30 ml/min and 34 cases of GIF. After 2008, there were only 36 GBCA exposures and no cases of GIF
Alhadad (Sweden)
2012
Gadodiamide
Gadopentetate
Gadoterate
Gadobenate
Gadoxetate
Gadoteridol
0/272
Retrospective chart review: 143 patients with stage 4 CKD and 129 with stage 5 CKD who had been exposed to one or more GBCAs
Amet (France)
2014
Gadoterate
0/255 (0 %)
Prospective cohort study: Assessment of dialysis patients getting GBCA. No cases of GIF were observed among those who received gadoterate (89 % of patients)
When a case of GIF occurred after administration of only a single GBCA product (regardless of exposure dose or number of individual exposures), it is considered to be an “unconfounded case.” In contrast, when GIF developed in a patient who received more than one GBCA product prior to disease onset, it is considered to be a “confounded case.” Almost without exception, unconfounded cases of GIF have been associated with formulated gadodiamide, gadopentetate dimeglumine, or gadoversetamide. In confounded cases, when GIF was associated with any other GBCA, the patient almost always also had been administered formulated gadodiamide, gadopentetate dimeglumine, or gadoversetamide prior to the onset of GIF. In 2010, based on these observations and the relative kinetic and thermodynamic stability of the various GBCAs, the FDA and the EMA both issued a risk stratification in which gadodiamide, gadopentetate dimeglumine, and gadoversetamide were classified as “high risk” for the development of GIF, whereas the other GBCAs were deemed to be “medium risk” or “low risk” (Fig. 15.1) [58, 59].
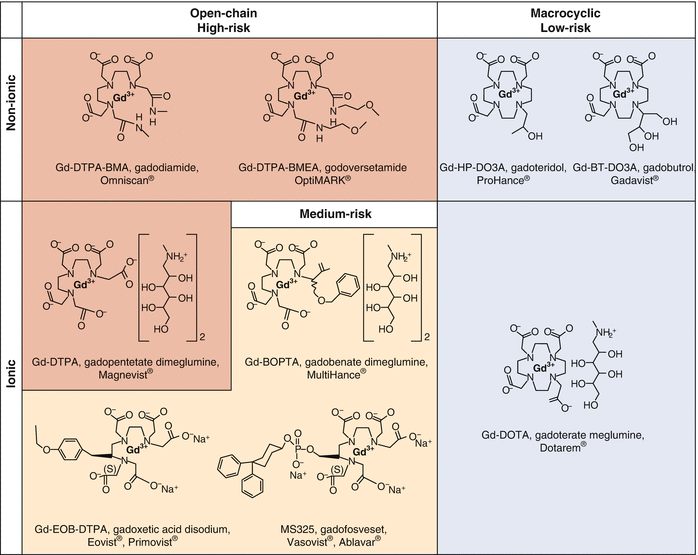

Fig. 15.1
Gadolinium-based contrast agents (GBCAs) categorized based on properties of structure (open chain and macrocyclic) and charge (ionic and nonionic). Shading indicates the categorization for risk of causing gadolinium-induced fibrosis (European Medicines Agency, 2010) (Modified from Bernstein and Kay [57] and reproduced with permission from Todd and Kay [23])
Many factors made it difficult for investigators to determine the strength of the association between GBCAs and GIF. Most importantly, retrospective chart review studies often depended upon documentation of GIF in the medical record by treating physicians, other than the investigators. In the early 2000s, GIF was largely unknown to the general medical community; many cases of GIF likely were never recognized or documented in studies that included this time frame. For this reason, many retrospective chart-based studies likely underestimated prevalence and incidence rates. Notably, a much higher prevalence of GIF was reported in both a cross-sectional cohort study [60] and a retrospective study [14] in which patients received their care from healthcare providers who were familiar with the GIF epidemic. Although many cases of GIF developed within days to weeks after exposure to a high-risk GBCA, some cases were not diagnosed until years after the GBCA exposure [10, 18, 31]. Thus, potential cases of GIF might not have been captured in a study if GIF was not diagnosed until after the dates studied.
An additional confounding factor to consider when interpreting epidemiologic studies of GIF is that patients with CKD may receive medical care at multiple healthcare institutions. Thus, a GBCA exposure might not be documented in the medical records available to investigators. This phenomenon has been observed in epidemiologic studies [39] and likely explains the very infrequent reports of patients who developed GIF with no known GBCA exposure [61–63]. Gd has been detected in biopsy tissue from GIF patients for whom GBCA exposure was not documented in available medical records [64]. Gd is not otherwise present in the human body so one can presume that tissue Gd must have resulted from prior GBCA exposure, even if none was documented in the record or recalled by the patient. One reason why investigators might overlook an exposure is that patients could have received the GBCA during an imaging study other than MRI, such as a fistulogram or another vascular imaging study [40].
Before 2006, the specific type and dose of GBCA were not consistently documented in medical records [39, 41]. Contributing to this was the perception that GBCAs were so harmless that their identification was not significant enough for inclusion in the medical record [65, 66]. Prior to Grobner’s 2006 publication, GIF was largely unknown to the radiology community [66]. Thus, investigators often had to rely upon institutional GBCA prescribing patterns to determine the brand and the dose of GBCA that was administered, making it difficult to show dose–response relationships between GBCA exposure and the onset of GIF in epidemiologic studies [39]. It is now standard practice (including at our respective institutions) that the specific type and dose of GBCA administered are included in the report of each GBCA-enhanced imaging study.
15.3 Histopathology and Pathogenesis
Histopathological assessment of GIF involves sampling affected tissue, which often requires a full-thickness skin biopsy that contains deep dermal and subcutaneous tissues. Biopsies from other organs can also be analyzed for evidence of systemic fibrosis. Regardless of the organ affected, several histological features are hallmarks of GIF. Reticular bundles of dermal collagen are thickened and surrounded by clefts, and dermal mucin is increased with intact elastic fibers (Fig. 15.2). CD68+ factor XIIIa+ dendritic cells and spindle cells that have a CD34+ CD45RO+ type I procollagen+ phenotype infiltrate the dermis [3, 5]. Lesions of established GIF are less cellular than those of early disease and contain abundant collagen. Dystrophic calcification is a well-recognized histologic characteristic of late GIF lesions, and osseous metaplasia and calcified sclerotic bodies have been suggested to be histologic features highly specific for GIF [4, 68, 69]. However, in the absence of characteristic clinical features, it may be difficult to distinguish GIF from scleromyxedema based only upon histopathological analysis [70].


Fig. 15.2
Skin biopsy from the leg of a patient with gadolinium-induced fibrosis demonstrating typical histologic changes: hypercellular dermis (hematoxylin–eosin stain, left) with thick and thin collagen bundles surrounded by clefts with spindle cells intercalated between the collagen bundles throughout the reticular dermis and extending into the septa of subcutaneous fat. These spindle cells are dermal fibrocytes that stain with antibodies to CD34 (right) (Reproduced with permission from Kay [67])
To better conceptualize the etiopathogenesis of GIF, it is important to understand the chemical and molecular properties that distinguish among the various GBCAs. Gd is a lanthanide series rare earth metal with seven unpaired electrons, which makes it highly paramagnetic and an ideal contrast agent for MRI. In solution, however, trivalent Gd+3 (“free gadolinium”) is highly toxic [71, 72]. There are multiple mechanisms by which Gd is toxic, including blockage of voltage-gated calcium channels [73], formation of inorganic Gd-phosphate precipitates [74], and induction of cytokines associated with tissue fibrosis (discussed later) [19].
To reduce the potential toxicity of Gd when used as a contrast agent, it is bound to an inorganic carrier molecule (“chelate”). It is this chelate that distinguishes among the various GBCAs approved for use in humans (Fig. 15.1). In 2006, five GBCAs were commercially available in the United States: formulated gadodiamide (gadodiamide with 5 % excess sodium caldiamide chelate, Omniscan®), gadoversetamide (OptiMARK®), gadopentetate dimeglumine (Magnevist®), gadobenate dimeglumine (MultiHance®), and gadoteridol (ProHance®). Additional GBCAs were available outside of the United States at that time or have become commercially available since 2006: gadoxetate disodium (Eovist®, Primovist®), gadofosveset trisodium (Vasovist®, ABLAVAR®), gadobutrol (Gadovist®), and gadoterate meglumine (Dotarem®).
GBCAs can be categorized by whether they have a linear or macrocyclic structure and whether they have an ionic or nonionic charge (Fig. 15.1). These two properties determine the kinetic stability and thermodynamic stability of each GBCA and largely explain the propensity for Gd to disassociate from its chelate to become toxic free Gd+3. Those GBCAs that are linear and nonionic (formulated gadodiamide and gadoversetamide) are the least stable. In contrast, macrocyclic GBCAs (gadoteridol, gadobutrol, and gadoterate meglumine), whether ionic or nonionic, are the most stable GBCAs. Intermediate in stability are those GBCAs that are linear but ionic (gadopentetate dimeglumine, gadobenate dimeglumine, gadoxetic acid disodium, and gadofosveset trisodium). These properties are noteworthy when considering that the vast majority of reported GIF cases developed after patients had been exposed to the least stable GBCA: linear nonionic formulated gadodiamide.
All GBCAs are large molecules that remain extracellular. Most are highly water soluble and excreted by the kidneys [75]. Two GBCAs (gadobenate dimeglumine and gadoxetic acid disodium) are more lipophilic, such that a sizeable fraction is excreted via the hepatobiliary system [76]. In patients with intact renal function, the half-life (t½) of formulated gadodiamide is 70 min: 95 % of a single dose is eliminated in the urine after 72 h [77]. However, the clearance of gadodiamide declines dramatically in patients with impaired renal function. The mean t½ of formulated gadodiamide is 34.3 h in non-dialysis-dependent patients with stage 5 CKD and 52.7 h in patients receiving CAPD [78]. Only an average of 68.0 % of formulated gadodiamide is cleared after one HD treatment, and only an average of 72.3 % of the original amount is removed after four HD sessions [78]. In patients with GFR <20 ml/min, <70 % of a single intravenous gadopentetate dimeglumine dose was recovered in urine by 48 h after administration [79]. Further, Gd tissue deposition has been observed in biopsies of multiple organs from patients with GIF, even years after the last known GBCA exposure [18]. Notably, in patients with normal renal function, Gd has been shown to deposit and accumulate in the brain and bone, with higher bone Gd content after the administration of formulated gadodiamide than of an equivalent dose of gadoteridol [26, 27, 80].
After their administration, GBCAs persist for many more hours in patients with advanced renal disease than in individuals with normal renal function. In patients with compromised renal function who have received a GBCA, free Gd is more likely to dissociate from its chelate and be released as Gd+3, especially with the less stable (high-risk) agents. Altered phosphate levels may affect the release of Gd+3 from unstable linear chelates, but published data are inconsistent [11, 81, 82]. When a GBCA dissociates, not only does it release free Gd+3 but also it releases the empty chelate, which then is able to associate with other cations, such as Fe+2 and Zn+2. This process is called transmetallation [83].
An early study described the toxicity of gadopentetate dimeglumine in 151 patients with renal disease [84]. Mostly, only mild adverse effects were reported. However, this was a retrospective chart review with short-term follow-up (30 days for outpatient MRIs or until hospital discharge for inpatient MRIs). Only 71 of the 151 patients (47 %) had serum creatinine values >2.5 mg/dL, and only 15 patients (10 %) were receiving HD. GIF had not yet been described in the medical literature at the time of this study. Thus, it is not surprising that neither cutaneous nor systemic fibrosis was reported in this relatively small study with a brief duration of follow-up.
Two additional factors must be considered regarding the use of GBCAs during the early 2000s. First, since its introduction in 1994 [85], GBCA-enhanced MR angiography was being performed with increasing frequency. In this procedure, patients often were administered twice or three times the standard 0.1 mmol/Kg dose of GBCA used for MRI [85]. Second, according to the FDA Office of Surveillance and Epidemiology (OSE), two GBCAs dominated the United States market during the mid-2000s: gadopentetate dimeglumine had an approximately 50 % share of the GBCA market, and formulated gadodiamide followed with a market share of almost 40 % [56]. The vast majority of patients who were reported to have developed GIF had been exposed to formulated gadodiamide, the least stable of the various GBCAs; many fewer patients were reported to have developed GIF after exposure to the slightly more stable GBCA gadopentetate dimeglumine. Thus, its thermodynamic instability, rather than its market share, likely accounted for the perceived greater propensity of formulated gadodiamide to cause GIF.
Many molecular, cellular, and animal studies have provided the framework to help understand the mechanism by which high-risk GBCAs likely cause GIF (Fig. 15.3). Many lines of evidence support the hypothesis that, with prolonged tissue exposure such as that which occurs in patients with significant renal dysfunction, formulated gadodiamide and other high-risk GBCAs dissociate into free Gd+3 and chelate [86]. Investigators have used microanalytical scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS), synchrotron X-ray fluorescence (SXRF) microscopy, extended X-ray absorption fine structure (EXAFS) spectroscopy, and inductively coupled plasma-mass spectrometry (ICP-MS) to characterize and to quantify Gd in insoluble deposits found in tissue obtained from GIF lesions [17, 18]. In these deposits, Gd+3 cations associate with complexes of inorganic phosphate, calcium, and sodium [87].


Fig. 15.3
Hypothesized pathophysiologic mechanism for gadolinium-induced fibrosis. In patients with renal dysfunction exposed to high-risk gadolinium-based contrast agents (e.g., formulated gadodiamide), free gadolinium is released into the tissues [86–89], where particulate Gd is internalized into macrophages by phagocytosis. These Gd particles are internalized into phagosomes that may rupture, releasing Gd into the cytosol, where it activates nuclear factor-κB [90] and preferentially stimulates M2 macrophages by way of the NLRP3 inflammasome [91]. This triggers production of pro-fibrotic cytokines [19, 90], which stimulate fibroblasts through tyrosine kinases [92] to upregulate the transcription, translation, and processing of extracellular matrix proteins [93, 94] (Reproduced with permission from Todd and Kay [23])
The amount of free Gd+3 released is the primary determinant of tissue toxicity [88]. Several factors favor the release of free Gd+3 from its chelate, including an acidic environment (as might be found in the phagolysosome of macrophages) [89] and transmetallation, whereby other cations, such as Fe+2, Zn+2, Ca+2, and Cu+2, may displace Gd+3 from its chelate [95]. The increased mobilization of iron that is observed after exposure of patients with dialysis-dependent renal disease to formulated gadodiamide provides evidence that transmetallation occurs in patients at risk for developing GIF [96].
In lesional tissue of GIF patients, mRNA for TGBβ1 (a pro-fibrotic cytokine) is increased [5]. In vitro, the release of free Gd+3 from GBCAs promotes the production of TGBβ1 and other pro-fibrotic cytokines, chemokines, and growth factors by monocyte-derived macrophages and peripheral blood monocytes [19, 90]. GBCAs also stimulate fibroblast proliferation and increased synthesis of extracellular matrix components, such as hyaluronic acid, fibronectin, and type I and III collagens [93, 94]. Formulated gadodiamide produces these pro-fibrotic effects at much lower concentrations than the more stable macrocyclic GBCAs [97, 98]. These effects are likely mediated by signaling through toll-like receptors (TLR) 4 and 7 [99] with activation of nuclear factor-kB (NF-κB) [90]. Free Gd+3, gadodiamide, and gadopentetate dimeglumine each activate the NLRP3 inflammasome in vitro to produce IL-1β; these molecules preferentially activate pro-fibrotic IL-4-polarized M2 macrophages [91].
Animal models of GIF demonstrate pro-fibrotic effects of various GBCAs, especially gadodiamide. Early studies, conducted before the first cases of GIF appeared, revealed toxic effects of GdCl3 in mice, but these were due mostly to mineral deposition in vascular and reticuloendothelial tissues [72]. Rats given high doses of gadodiamide developed skin ulceration, an effect that was attributed to abnormal zinc metabolism [100]. After the association between GBCAs and GIF was recognized, investigators revisited animal models, administering GBCAs intravenously to rats that had or had not undergone partial nephrectomy [20, 21, 101, 102]. Although animal models do not recapitulate human GIF perfectly, several common themes emerged from these studies. First, formulated gadodiamide induced cutaneous changes in rats that were similar histologically to those of GIF, displaying increased cellularity and tissue fibrosis with increased collagen deposition and dermal thickening. Second, formulated gadodiamide was the only one of the eight GBCAs tested that induced these changes to any significant degree. Third, among rats that had received GBCAs, the highest tissue concentrations of Gd were detected in tissues of those that received formulated gadodiamide [20, 101, 102]. TGF-β1 was detected in lesional skin from rats treated with gadodiamide but not from those treated with gadoteridol [101], as it had been in the skin of patients with GIF [5].
Previously, we and others have applied Bradford Hill criteria [28] to contend that the relationship between high-risk GBCAs and GIF is not just an association but rather implies causation [103–106] Todd and Kay [23]. We readdress this line of reasoning in Table 15.2, updating the nine criteria with additional data presented in this review. With regard to strength of association, a meta-analysis of seven epidemiologic studies calculated an odds ratio of 27 for the association between GBCA exposure and the subsequent development of GIF [103], which is comparable to the odds ratio associating heavy cigarette smoking with lung cancer [28]. Consistency also has been fulfilled, since many investigators working at different institutions in different countries have each identified similarly strong associations between exposure to high-risk GBCAs and GIF [8, 10, 11, 14, 31]. Specificity is fulfilled by the absence of convincing cases of GIF occurring without known GBCA exposure; Gd has been detected in lesional skin even from patients with GIF who recalled no prior GBCA exposure [64].
Table 15.2
Bradford Hill criteria for causation
Criterion | Supporting evidence |
|---|---|
Strength of association | Very strong odds ratio associating GBCA exposure and GIF |
Consistency | Many independent investigators from different institutions and different countries have found a similarly strong association |
Specificity | GIF not proven to occur in the absence of GBCA exposure |
Temporality | GBCA clearly precedes the onset of GIF, sometimes by years |
Biological gradient | Many studies have shown a greater risk of GIF with exposure to higher doses of or repeated exposures to GBCAs |
Biological plausibility | Many lines of molecular, cellular, and animal data support the “free gadolinium” hypothesis that Gd+3 in high-risk GBCAs dissociates from its chelate to trigger a cascade of events that result in GIF |
Coherence | GIF has not been shown to result from any exposure other than to GBCAs |
Experiment | Animal exposed to formulated gadodiamide develop a fibrosing condition that resembles GIF Avoiding use of GBCAs in patients with renal dysfunction has essentially eliminated incident cases of GIF |
Analogy | Historically, environmental toxins caused Spanish toxic oil syndrome and eosinophilia-myalgia syndrome |







