, Muhammad Shamim Khan1 and Prokar Dasgupta2
(1)
Department of Urology, Kings College London, Kings Health Partners, Guy’s Hospital, Great Maze Pond, London, UK
(2)
Kings College London, Kings Health Partners, Guy’s Hospital, MRC Centre for Transplantation NIHR Biomedical Research Centre, London, UK
Abstract
Radical cystectomy is currently accepted as the standard of care for muscle-invasive (MIBC) and high-risk non-muscle-invasive bladder cancer (NMIBC) and nowadays can be performed with open, laparoscopic or robotic approach.
The robotic approach has become increasingly popular; thanks to better ergonomics, improved higher magnification 3D vision and providing the surgeon the independence of controlling the camera and multiple instruments, which can reproduce the whole spectrum of movements of a human hand. This has made the most difficult steps of intra-corporeal knotting and suturing easier and quicker than laparoscopy. In this chapter we analyse the possible future directions of robotic surgery for urinary bladder cancer and we focus our analysis on surgical access and instrumentation, techniques of urinary diversions, simulation and training and future employment of chemical agents which could improve the detection rate of a early nodal metastasis.
Introduction
Radical cystectomy is currently accepted as the gold standard for treatment of MIBC and high risk NMIBC. This procedure can be performed using open, laparoscopic or robotic approach.
In 1991 R. Clayman performed the first laparoscopic radical nephrectomy and became the pioneer of the new era of minimally invasive surgery in urology. During the last 20 years, indications for laparoscopic surgery have expanded to include both ablative and reconstructive procedures for various indications. Its place in the armamentarium of urological and other surgical specialties is well established.
Laparoscopic surgery, however, generally has longer operative times than open surgery and requires a steeper learning curve due to the need for the surgeon to develop high skills for intra-corporeal suturing and knotting; abilities which are required in the majority of urological procedures. These characteristics have been identified as the main limiting factors for widespread adaptation of laparoscopic surgery in urology. While many urology centres could not progress in technical expertise to venture starting technically challenging procedures like partial nephrectomy and radical prostatectomy, others suspended these programmes in favour of a standard open surgical approach [1].
In 2001, the era of robotic surgery (RS) began with the introduction of the Da Vinci surgical system® (Intuitive Surgical, Inc., Sunnyvale, California, USA, http://www.intuitivesurgical.com).
RS represents a further evolution of laparoscopy with better ergonomics, improved, higher magnification 3D vision and providing the surgeon the independence of controlling the camera and multiple instruments, which can reproduce the whole spectrum of movements of a human hand. This has made the most difficult steps of intra-corporeal knotting and suturing easier and quicker than laparoscopy. RS has become increasingly popular and, at many centres around the world, robotic radical prostatectomy has essentially become the standard of care for a localised prostate cancer.
As regards radical cystectomy, there is evidence that robot-assisted radical cystectomy (RARC) provides outcomes similar or even superior to laparoscopic and open radical cystectomy. However, long-term follow-up data are still lacking. There is a significant heterogeneity in the data reported in the literature in relation to approaches to urinary diversion.
In this chapter we analyse the possible future directions of robotic surgery for urinary bladder cancer with focus on our analysis of surgical access and instrumentation, techniques of urinary diversions, simulation and training and future employment of chemical agents which could improve the detection rate of a early nodal metastasis.
Single-Site Surgery and Instrumentation
Laparoendoscopic single-site surgery (LESS) and natural orifice transluminal endoscopic surgery (NOTES) are relatively novel techniques in urology and have gained prominence in academic centres which have a strong interest for laparoscopic surgery.
NOTES can be defined as the penetration of hollow viscera with an endoscope in order to access the abdominal cavity and perform an intra-abdominal operation [2]. On the contrary, a LESS access can be obtained either by performing a single incision on the skin, through which a single multi-channel access platform is placed (single port) or by placing several low-profile ports through separate fascial incisions (single site) [3–5]. Single-port access is used most commonly, and there are several commercially available ports, including Triport and Quadport, Advanced Surgical Concepts, Ireland (see Figs. 20.1 and 20.2). The umbilicus is the most frequently chosen access site.
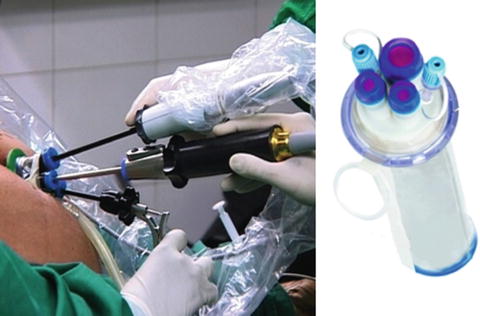
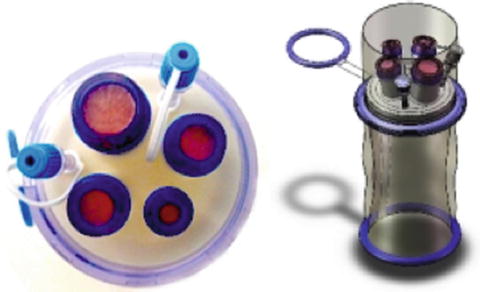

Fig. 20.1
Triport (R-Port), Advanced Surgical Concepts, Ireland. http://www.advancedsurgical.ie/TriPort/Default.166.html

Fig. 20.2
Quadport, Advanced Surgical Concepts, Ireland. http://www.advancedsurgical.ie/QuadPort/Default.544.html
The lack of port placement triangulation and collision of instruments make LESS a challenging technique and requires an experienced laparoscopic surgeon. These tasks become even more demanding during reconstructive procedures when suturing is needed, and as a result instruments have been especially conceived to facilitate LESS. Application of robotic technology for single-port surgery has enhanced intra-corporeal dissection and suturing, albeit the platform can be cumbersome. Therefore further refinements will need to occur before full utilisation of LESS.
LESS radical cystectomy (RC) has been attempted in highly selected patients, with or without robotic assistance and so far appears safe and feasible. Kaouk et al. were the first to report LESS robot-assisted radical cystectomy and bilateral pelvic lymph node dissection (PLND) in three patients (two men and one woman). All procedures were completed successfully and all patients underwent extracorporeal urinary diversion by extending the umbilical port site. The operative time was 315 ± 40 min, with minimal blood loss (217 ± 29 mL). The pathologic evaluation revealed negative margins and negative lymph node involvement (mean number of nodes 16 ± 3). At a minimum of 2 years of follow-up (range 24–26 months), no evidence of local recurrence or metastatic disease was detected.
The same group has subsequently performed three robot-assisted LESS (R-LESS) radical cystectomy with intra-corporeal ileal conduit [6]. The Authors inserted an additional trocar that served as the stoma site. None of these were converted to open, although one rectal perforation in a patient who had previous brachytherapy was identified and repaired by R-LESS. Within a mean follow-up of 12.6 months (8–18), one patient developed deep vein thrombosis (DVT) and was managed medically. No patients had positive surgical margins on final histology.
Lin et al. described “hybrid-LESS” radical cystectomy with orthotopic neo-bladder reconstruction in a cohort of 12 patients [7]. A homemade multi-channel port, consisting of two stretchable rings and a surgical glove with trocars and valves attached to its fingers, was placed into a 4–5-cm midline incision in the lower abdomen and was used for laparoscopic instruments (see Figs. 20.3 and 20.4). Another sub-umbilical port was placed for the laparoscope. Extended pelvic lymph node dissection and radical cystectomy were completed laparoscopically; construction of the ileal neo-bladder was performed extra-corporeally and the neo-bladder was anastomosed to the urethral stump laparoscopically, with a slipknot running suture technique. No conversion to standard laparoscopy or open surgery was required. There was no peri-operative mortality or port-related complications. Median operative time was 383 min (300–447) with median blood loss of 150 mL (120–400). Encouragingly, this group was able to retrieve a median of 25 lymph nodes (16–30), also with negative surgical margins. Continence rates were 80 % at 12-month follow-up. All patients were alive and tumour free at average follow-up of 16.1 months (range 9–20 months).
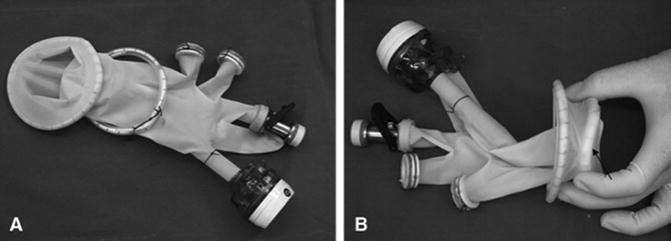


Fig. 20.3
(a) A homemade multichannel port was constructed of two stretchable rubber rings and a surgical glove. (b) The outer and inner rings of the multichannel port (arrow indicates the inner ring). Lin T et al. J Endourol. 2011 Jan;25(1):57–63

Fig. 20.4
(a) The incision and multichannel port. (b) The multichannel port was placed into the incision. (c) Insufflation with CO2 created tension between the two rings and secured the device
Ma et al. [8] also performed five LESS radical cystectomies using a homemade single-port device composed of an inverted cone device of polycarbonate and a powder-free surgical glove (see Fig. 20.5). The port was placed into a 5-cm peri-umbilical incision. The conventional laparoscope and laparoscopic instruments were inserted through the single port. No additional ports were needed for radical cystoprostatectomy and standard pelvic lymph node dissection. Cutaneous ureterostomy and ileal conduit urinary diversion were performed. The mean operative time was 208.2 (range 168–280 min) and estimated blood loss was 270 (100–500) mL. One patient needed a transfusion of 400 mL of red blood cells. The pathologic evaluation revealed negative margins and negative lymph node involvement. One patient had a bowel obstruction, while another patient died from cardiac disease.


Fig. 20.5
Home-made single-port device. Ma LL et al. J Endourol. 2012 Apr;26(4):355–9
Undoubtedly an extensive experience in laparoscopic surgery and stringent patient-selection criteria are fundamental for LESS radical cystectomy. Based on the available non-randomised evidence, these methods can be best regarded as experimental and hence strictly limited to centres with high volume laparoscopic, robotic and LESS practice.
All the studies discussed above are retrospective and are small case series (<20), with the majority having ≤T2 disease. Randomised prospective studies are needed to determine how this technique compares with the traditional open radical cystectomy (ORC) or multi-port robot-assisted radical cystectomy (RARC). Generally RC is often undertaken in an elderly population where cosmesis is not usually a priority, so there needs to be careful consideration as to whether this avenue should be pursued any further. It is possible that a further expansion of the indications and role of single-site surgery may occur with refinement of robotic technology, particularly, the development of more flexible instruments and this would influence our future surgical practice.
Currently available robotic instruments for LESS robotic surgery (LESS-RS) are 5 mm in size, non-wristed and semi-rigid. These instruments (see Fig. 20.6) can be inserted into the abdominal cavity through a silicone port (see Fig. 20.7) which requires only a 2–2.5-cm skin incision. The trocars for the Da Vinci® single-port system are curved and marked with the same remote centre of motion (RCM) that can be found on each trocar used for a standard Da Vinci® procedure. In fact, each robotic instrument is able to rotate around a specific epicentre (RCM), marked as a black thick line on the port. The RCM provides a wide range of motion to operate the instruments and it is always located slightly lower to the level of the skin. In this way, the risk of collision between the instruments and the body of the patient is avoided. Thanks to the presence of the RCM and to the fact that the instruments are actually crossing within the silicone port, and not outside the body (as on the contrary happens during single port laparoscopic surgery), the risk for a collision of the instruments inside the abdomen is minimised.

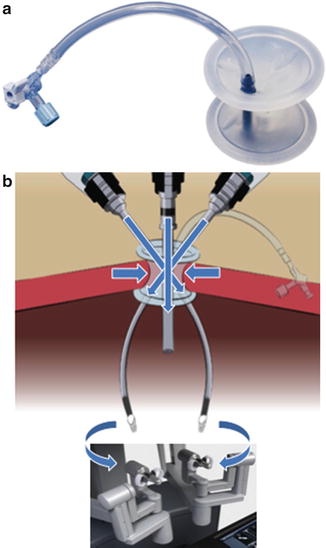

Fig. 20.6
Instruments for Single Port RS Da Vinci. Instruments are 5 mm in size, non wristed and semi-rigid. Intuitive surgical, Sunnyale, CA

Fig. 20.7
(a) Silicon port for Single Port RS Da Vinci (b)
However, knotting and suturing in LESS-RS is more difficult than in standard RS. The main limitation of the Da Vinci® Si system for LESS-RS is the lack of the endo-wrist technology. The presence of a wrist at the tip of the instrument allows the surgeon to perform movements with 7° of freedom. This feature facilitates manoeuvres such as intra-corporeal dissection, knotting and suturing.
Recently new 5-mm instruments with endo-wrist capability have been released for the Da Vinci® Si system (see Fig. 20.8). These have been used for paediatric robotic surgery but are also suitable for standard RS. It is expected that in the future, LESS-RS will be performed with miniaturised instruments characterised by multiple joints, high flexibility and wristed tip.


Fig. 20.8
5 mm endo-wrist instruments for Da Vinci
Research into microscopic and miniaturised technology in clinical practice has been underway in the last decade. Miniature camera robots (microrobots) can provide a mobile viewing platform to enhance a surgeon’s view, and nano-robots are also approaching clinical application. Interestingly, “in vivo robots”—which are miniature, dexterous, co-operative robotic devices can be deployed intra-corporeally to perform tasks such as imaging, retraction and tissue manipulation to enable precise movements [9]. These are the exciting developments and we eagerly await the outcome of research into their use in the oncological setting.
The future of RS will depend on the availability of new enhanced robotic systems. The current Da Vinci® Si system for RS is far from perfect and has several limitations:
The robotic cart is still cumbersome. Although the arms are smaller in size compared to the original Da Vinci® (see Fig. 20.9), they are still quite heavy and are all attached to a cart. The cart itself is quite heavy and is not easily manoeuvred by the nurses. Development of lighter arms that could be attached independently to the operative table has been advocated as a possible solution to ease and reduce the time for docking.
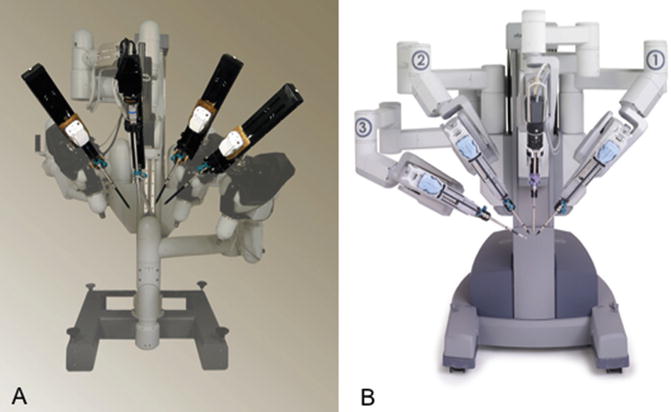
Fig. 20.9
(a) Da Vinci Standard patient Chart, 1999; (b) Da Vinci S and SI patient chart
The robotic camera could be miniaturised. A miniaturised magnetic camera that could be inserted into the abdominal cavity through a 5-mm port would allow the surgeon to manoeuvre the camera from the outside by using a calamite. This would avoid the need of a port dedicated to hold the camera.
The lack of tactile feedback is a significant deficiency of the system. Despite the presence of the endo-wrist technology, the robotic instruments are not able to transfer a tactile feedback to the hands of the surgeon who is operating at the console. This can be potentially dangerous. In fact, the accidental clashing of an instrument with an organ may result in a severe injury that could be avoided if the surgeon had the feeling of touching the tissues. However, it is possible to replace the lack of tactile feedback with an intensive training in the dry-lab.
The number of instruments available is still low. Despite the recent introduction of new instruments such as the Graptor, an 8-mm grasping retractor that allows atraumatic grasping of bowel and other delicate tissues, there are no instruments dedicated to specific procedures. As far as RARC is concerned, there is no miniaturised robotic stapler designed to perform an intra-corporeal diversion and there is no retractor specifically designed for RARC.
Intracorporeal Urinary Diversion
The majority of surgeons performing RARC recommend a combination of robot-assisted extirpative cystectomy and lymph-adenectomy, with subsequent extracorporeal urinary diversion, whether this be ileal conduit, orthotopic neo-bladder (ONB) or continent cutaneous diversion (CCD) formation [10–13]. As experience with RARC increases, intra-corporeal reconstruction of urinary diversion are emerging, in the expectation that this approach may confer benefits such as lesser incisional pain, with decreased bowel exposure and desiccation, thereby reducing the risk of ileus and resulting in faster recovery.
Approaches to reconstruction include extra-corporeal ONB, re-docking of the robot with robot-assisted anastamosis of urethra to ONB and total intra-corporeal reconstruction. The intra-corporeal approach is thought to be ergonomically beneficial in ONB formation, because the depth perception and optical magnification of the robot system facilitates formation of the anastamosis between urethra and reservoir. This minimises the risk of urinary leakage and may improve urinary continence [14].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





