and Christopher Isles2
(1)
Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK
(2)
Dumfries and Galloway Royal Infirmary, Dumfries, UK
We have included a separate chapter on fluid management because most patients with acute kidney injury are dry requiring fluid replacement and many will be septic, requiring fluid resuscitation.
Q1 Describe the different body fluid compartments
Total body water makes up around 60 % of total body weight. This means that for a 75 kg person body water will be about 45 litres. Two third of this (30 litres) is intracellular and one third (15 litres) is extracellular. Two thirds of the extracellular fluid (approximately 10 litres) is interstitial and 1/3 (approximately 5 litres) is intravascular (plasma). The three body compartments are separated by membranes with different permeabilities: both are freely permeable to water but only capillary membranes are freely permeable to electrolytes (Fig. 17.1).
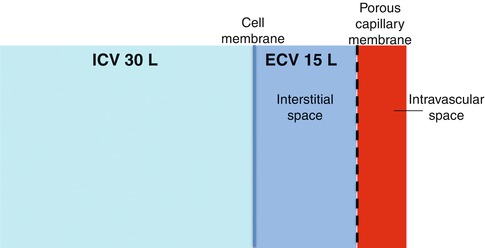

Fig. 17.1
Body fluid compartments. ICV intracellular volume, ECV extracellular volume
Q2 What is normal fluid intake and output?
Normal intake is around 2,000 ml/day and normal output 1,500 ml urine plus 500 ml insensible losses. Healthy individuals can of course cope with higher and lower intakes, principally by the effects of aldosterone and ADH on urine output. Medical students who feel they need to sip water from a plastic bottle on ward rounds in order to keep themselves adequately hydrated can be reassured!
Q3 Name three things that can go wrong with fluid balance?
There may be an imbalance between input and output, redistribution of fluid or an osmolar problem. Imbalance means either too much or too little fluid and is the easiest of the three to measure. Redistribution occurs as a result of leaky capillaries. This is driven by large plasma proteins especially albumin which is responsible for keeping fluid inside capillaries in health and which leaks out into the tissues taking fluid with it when a patient becomes unwell. Osmolar problems across cell membranes including the blood brain barrier are driven by the concentration of small diffusible ions, mainly Na, K, glucose and urea. Together these ions exert a ‘pressure’ which causes water to move across cell membranes from weaker to stronger solutions until the concentration of solutes is equal on both sides. The classic example of this is cerebral oedema which occurs when water moves into the brain e.g. in acute hyponatraemia.
Q4 How might you decide whether a patient is dry, wet or euvolaemic?
A patient with vomiting, diarrhoea, excessive stoma losses or inadequate fluid intake is almost certainly going to be dry, but in the absence of such obvious clues assessment of fluid volume status is often trickier than it looks. No single sign is sufficiently sensitive and specific to confirm or exclude hypovolaemia and clinical assessment must be interpreted in context of history and relevant lab results. The signs shown in the box below have been taken from the Academy of Royal Colleges AKI Competency Framework. A postural rise in pulse and fall in blood pressure may be detected by sitting a patient up rather than asking them to stand, if too unwell to do so. Other clues to dryness include a urea:creatinine ratio >100:1 (normal range is 60–80:1). Urine/plasma osmolality greater than 1.1 and urine sodium <20 mmol/l also suggests dryness though these tests have little added value in most patients. CVP measurement does not have a role in the routine assessment of volume status.
Box 17.1 Assessment of Fluid Volume Status
1.
Postural changes in pulse and blood pressure*
2.
Dryness of axillae*
3.
Moistness of mucous membranes
4.
Skin turgor
5.
Jugular venous pressure
6.
Capillary refill time
7.
Changes in body weight
8.
Urine output and fluid balance chart
9.
Peripheral or pulmonary oedema, pleural effusions, ascites
10.
Physiological response to a fluid challenge*
*said to be the most useful signs of dryness
Q5 Is it possible to be intravascularly dry and yet oedematous at the same time?
Yes. Redistribution of fluid as a result of leaky capillaries in a septic patient is perhaps the commonest clinical scenario. Patients with massive leg oedema and/or ascites may well be dry intravascularly as indeed can occasional patients with pulmonary oedema (the classical scenario being right ventricular infarction). It is of course always more difficult to assess for dryness in a patient who is ‘wet’ and such patients are certainly more difficult to manage than those without oedema, simply because the underlying problem cannot usually be corrected by giving large volumes of intravenous fluid. The combination of cardiac and renal failure is often called cardiorenal failure, which we discuss in more detail in Chap. 12.




Q6 How might you calculate how much fluid to give?< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








