Flaps (Excision and Closure, Mucosal, Skin)
M. Solomon
C. Wright
Introduction
The management of complicated anal fistula disease remains a challenge for surgeons and a frustrating problem for patients. Treatment is aimed at cure, with the drainage of associated sepsis and eradication of the fistula tract, while at the same time preserving the integrity of the anal sphincter and continence.
The majority of perianal sepsis is idiopathic or cryptoglandular in origin, and arises from the obstruction of the anal glands, leading to stasis of glandular secretions and, if secondarily infected, suppuration and abscess formation. The abscess typically forms in the intersphincteric space but can extend into the ischiorectal fossa or supralevator/suprasphincteric spaces. A fistula tract may subsequently form.
A minority of cases are secondary to an underlying disease process, including Crohn’s disease, local radiation, malignancy, trauma, tuberculosis, HIV, hidradenitis suppurativa, lymphogranuloma venereum, perianal actinomycosis, and rectal duplication. Rectal and foreign body trauma should also be considered as possible etiological causes. In these situations, the fistula tract is often atypical.
All methods of fistula repair rely on the elimination of the internal opening to the anal gland.
Fistulotomy remains the mainstay of treatment for those fistulas involving a small amount of sphincter muscle such as low transsphincteric and intersphincteric fistula tracts. The challenge lies in the treatment of complex fistula tracts. In this situation, a simple fistulotomy is precluded because the division of sphincter muscle would result in an unacceptably high rate of incontinence. As a result, techniques that preserve sphincter function have been developed. These include simple excision and closure of the internal opening, the ligation of intersphincteric fistula tract (LIFT) procedure, advancement flap repairs, and more recently, the use of biological materials to occlude the tract such as fibrin glue and fistula plugs.
Excision and Closure of the Internal Opening
Some authors have reported treating the tract by simply closing the internal fistula opening, without using an advancement flap. The argument for this type of repair is that in the presence of adequate perfusion and the absence of undue tension, at the coapted surfaces, simple appositional closure should suffice (1). The repair maintains the sphincter integrity. This procedure is not practiced at our institution, as it has been shown to appear to be inferior to the methods using flap reinforcement, and is generally now combined with either a flap advancement procedure and/or a fistulotomy. The more recently described modification, the LIFT procedure (discussed in detail in Chapter 9, pages 79–84), which involves ligating the intersphincteric fistula tract, may change this attitude.
Flaps
Advancement flap procedures may be considered in any patient in whom the fistula tract is complex and cannot be laid open. A complex tract is defined as a tract that crosses >30–50% of the external sphincter and includes those tracts which are high transsphincteric, suprasphincteric, and extrasphincteric; rectovaginal or rectourethral; anterior in female patients; multiple or recurrent; or the patient has preexisting sphincter compromise. In addition, the tract is complex if it is associated with an underlying disease process, as listed above, including Crohn’s disease.
The flap may be mobilized from the rectum as either a mucosal, partial thickness or full thickness rectal advancement flap or from the perianal skin as an anocutaneous advancement flap.
Relative contraindications to performing a flap repair include:
undrained sepsis
a fistula of less than 4 weeks duration
a malignant fistula
a fistula arising in an irradiated field
the presence of active proctitis, particularly Crohn’s disease
An anorectal stricture would be a relative contraindication to performing a mucosal rectal advancement flap and an anocutaneous flap would be preferably used in this situation. An anocutaneous advancement flap is also used to repair keyhole deformities related to sphincter defects and scarring as a result of previous fistulotomies.
Understanding the anatomy of the pathology is a prerequisite for successful treatment.
Preoperative evaluation and planning determines the course of the tract in relation to the sphincter complex with identification of the internal opening, the secondary or external opening, the primary tract, and any secondary extensions of the tract. Baseline sphincter function should also be assessed and any coexisting disease should be identified. Clinical assessment is aimed at identifying any symptoms which suggest coexisting disease and assessing a patient’s baseline level of continence. A past history of previous anal surgery and an obstetric history is also sought.
Digital examination and rigid sigmoidoscopy remain an essential part of the preoperative assessment and are often the only investigations required for simple fistulae. The internal opening may be felt as a palpable defect and the course of the tract is indicated by induration of the perianal tissues. The site of the external opening is usually clear. Digital examination is also a guide to sphincter function. Prior to more invasive tests the anal resting tone and voluntary sphincter “squeeze” pressure are also assessed clinically. Sphincter defects may be palpable and, deformity of the anal canal due to anorectal sepsis or previous surgery, is noted.
The findings often influence the choice of operative procedure. In the presence of anorectal stenosis, and/or extensive scarring and rigidity of the rectal mucosa as a result
of chronic suprasphincteric sepsis for example, an anocutaneous flap repair is preferred because of the potential difficulty in mobilizing a rectally based advancement flap in this situation.
of chronic suprasphincteric sepsis for example, an anocutaneous flap repair is preferred because of the potential difficulty in mobilizing a rectally based advancement flap in this situation.
It is the authors’ practice to perform a colonoscopy or flexible sigmoidoscopy for complex or recurrent fistulae, to help exclude associated gastrointestinal disease. The choice of investigation usually depends on the patient’s age and whether or not they have the associated abdominal symptoms. If Crohn’s disease is suspected on symptoms, and a colonoscopy is normal, then an upper endoscopy, small bowel series, enteroclysis, small bowel enteroscopy, or small bowel capsule imaging may be indicated.
The complex fistula tract may be imaged by endoanal ultrasound (EAUS) or magnetic resonance imaging (MRI). Fistulography and computed tomography (CT) have little to offer in the assessment of the fistula tract. The choice of imaging is dependent on availability, local expertise, and the complexity of the fistula tract. It has been the authors’ practice to assess recurrent and complex fistula tracts with 2D EAUS and, more recently, using 3D US reconstruction.
EAUS is able to differentiate between simple and complex fistulae, detect abscesses, delineate the internal and external openings, and categorize the type of tract. Hydrogen peroxide injected via the external opening can enhance visualization of the tract as it acts as an ultrasonic contrast medium by producing hyper reflective gas bubbles (2). Although the use of hydrogen peroxide has been shown to increase the accuracy of assessment from 68–98%, it has not been our practice to need or to use enhancement routinely. EAUS facilitates surgical planning as well as confirming previous sphincter damage as a result of the disease process and/or previous surgical attempts at eradication. An advantage of EAUS over MRI is that EAUS can be performed during surgery as an adjunct to digital examination and examination under anesthesia (EUA) as well as provide a dynamic preoperative assessment of sphincter integrity.
In Australia, currently, MRI has limited availability and remuneration; it is also more time consuming, expensive, and not available as an intraoperative resource. It is, however, considered as the gold standard for assessment of anal sepsis by some surgeons. MRI is more accurate than clinical assessment in detecting previously missed secondary extensions of the primary tract, and in the correct assessment of the level of the fistula with respect to the sphincter complex. The use of endocouple receivers increases tissue resolution in close proximity to the anal canal, providing superior anatomical detail. Pelvic phased array coils can be used to assess supralevator or more extensive sepsis.
Both, EAUS and MRI, are associated with a learning curve in the interpretation of the images.
The internal sphincter is particularly at risk in fistula surgery, and baseline anal manometry to measure the anal canal pressures can also influence the choice of surgical technique.
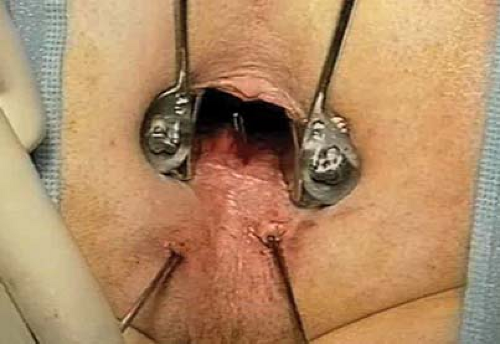
Prior to definitive repair surgery an EUA to further assess the tracts is sometimes useful. The authors prefer to use Lockhart Mummery probes (Fig. 5.1) to the smaller
caliber lacrimal probes because of the greater risk of creating a false tract with the latter.
caliber lacrimal probes because of the greater risk of creating a false tract with the latter.
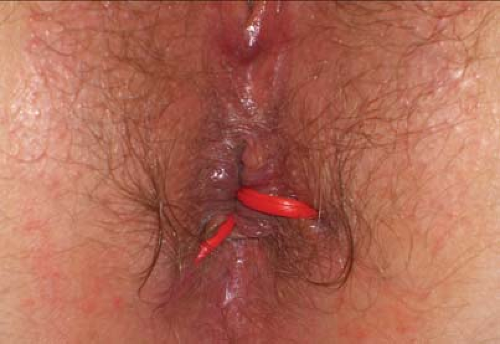 Figure 5.2 Two quiescent fistula tracts are demonstrated, with sepsis controlled following the placement of seton drains (vessel loops). |
Definitive repair should only be undertaken after sepsis has been controlled, i.e. abscesses have been incised and drained and there has been a period of controlled drainage by placement of loose vessel loop seton drains (Fig. 5.2). Drainage from large chronic supralevator cavities is best controlled with small mushroom tip (de Pezzer) drains.
Perioperative Management
It is the authors’ preference to use a sodium phosphate enema to clear the distal bowel. A full mechanical bowel preparation is rarely required.
Informed consent is obtained. The risks specific to fistula surgery that should be explained to the patient include:
the risk of failure/recurrence (see results)
inadvertent, or greater than expected, compromise of sphincter function and incontinence
anorectal sepsis
hematoma formation
iatrogenic fistula formation including rectovaginal fistulae
ectropion (following mucosal advancement flaps)
Positioning
In the operating theatre, consent and the procedure to be performed are checked by the operating surgeon prior to the commencement of the procedure. The patient’s medical history is reviewed to check for any allergies to drugs that may be used. If the fistula tract is posterior or lateral then the patient is placed in the lithotomy position; some prefer the prone jackknife position for anterior tracts. The assistant is better positioned and more comfortable when the patient is prone.
The procedure is usually performed under general anesthesia using a laryngeal mask without muscle relaxation. Regional anesthesia with intravenous sedation is indicated on occasions because of patient morbidity. Antibiotic prophylaxis is routine, the authors’ preference being the combination of gentamicin and metronidazole or a third generation cephalosporin and metronidazole. Thromboembolic prophylaxis is guided by the patient’s age, weight and any associated comorbidities, and the estimated length of the procedure but subcutaneous heparin is used routinely.
The perineum, including the vagina and anal canal, is prepped with aqueous chlorhexidine and square draped. Fenestrated drapes should be avoided because they tend to shift and limit the access. Use of a headlight can optimize the view but is not usually necessary. When performing an anocutaneous flap the perianal buttock area is shaved if it is hirsute; an indwelling urethral catheter is not usually necessary.
Technique
A confirmatory EUA is performed and definitive repair is postponed if there is evidence of residual sepsis.
Retraction is important, so the buttocks are taped apart in the prone position. The Lone Star retractor alone, or together with either a Park’s anal, bivalve retractor (Eisenhammer) or Hill-Ferguson retractor, provides adequate exposure.
Simple Excision and Appositional Closure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree