Introduction
Female urology encompasses urinary incontinence as well as pelvic reconstructive medicine for prolapse. It is common for urinary incontinence and pelvic organ prolapse to coexist in the same woman or to develop subsequently. This chapter will concentrate on pelvic organ prolapse as urinary incontinence has been discussed in detail in Chapter 29.
Pelvic organ prolapse is the protrusion of the pelvic organs (uterus, bladder, and bowel) into or past the vaginal introitus. Estimates of prevalence vary widely depending on definition used, whether the patient is symptomatic, the epidemiologic methods used, and the population studied. The U.S. National Center for Health Statistics estimates over 250,000 operations performed for genital prolapse apart from hysterectomy. With aging of the population, these quality of life issues and their treatment assume additional importance.
Anatomy
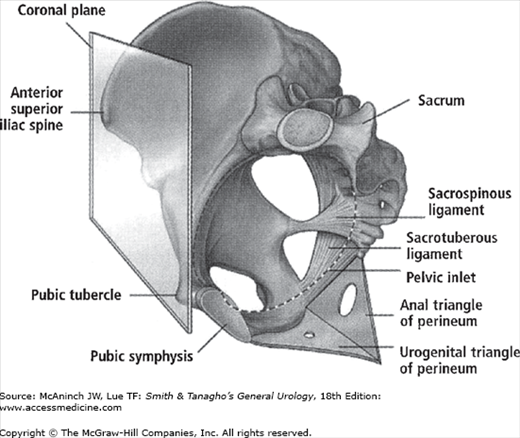
The maintenance of continence and prevention of pelvic organ prolapse rely on the support mechanisms of the pelvic floor. The bony pelvis is the rigid foundation to which all of the pelvic structures are ultimately anchored. These bones are the ilium, ischium, pubic rami, sacrum, and coccyx. It is important to understand and discuss the bony pelvis from the perspective of a standing woman. In the upright position, the bony arches of the pelvic inlet are oriented in an almost vertical plane (Figure 40–1). This directs the pressure of the intra-abdominal and pelvic contents toward the bones of the pelvis instead of the muscles and fascial attachments of the pelvic floor. This dispersion of forces minimizes the pressure on the pelvic musculature and transmits them to the bones that are better suited to the long-term cumulative stress of daily life.
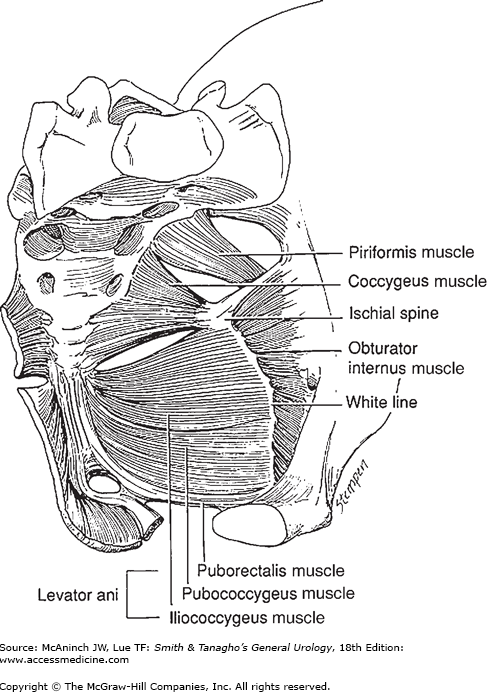
The muscles of the pelvic floor, particularly the levator ani muscles, have a critical role in supporting the pelvic organs and play an integral role in urinary, defecatory, and sexual function. The levator muscle complex consists of the pubococcygeus, the puborectalis, and the iliococcygeus (Figure 40–2). The pubococcygeus originates on the posterior inferior pubic rami and inserts on the midline organs and the anococcygeal raphe. The puborectalis also originates on the pubic bone, but its fibers pass posteriorly and form a sling around the vagina, rectum, and perineal body, resulting in the anorectal angle and promoting closure of the urogenital hiatus. The iliococcygeus originates from the arcus tendineus levator ani (ATLA) and inserts in the midline onto the anococcygeal raphe.
The ATLA is a linear fascial covering of the obturator internus muscle, and extends from the ischial spine to the posterior area of the superior ramus (Figure 40–2). The levator ani muscle group forms a broad hammock upon which the bladder, proximal vagina, and intrapelvic rectum lie, providing the musculofascial support for a large portion of the anterior pelvis. The space between the levator ani musculature through which the urethra, vagina, and rectum pass is called the urogenital hiatus. The fusion of levator ani where they meet in the midline creates the so-called levator plate.
The pelvic diaphragm has investing connective tissue, which is often referred to as “fascia”; it is, however, less organized and less distinct than traditional fascia (eg, rectus abdominis fascia). This visceropelvic fascia consists of collagen, smooth muscle, and elastin. Microscopic studies suggest that this fascia may be histologically indistinct from the deep vaginal wall, and not a separate “fascia.”
The pelvic fascia consists of two leaves—the endopelvic fascia (abdominal side) and the perivesical fascia (vaginal side). The urethra, bladder, vagina, and uterus are all contained within these two layers of fascia. The two leaves fuse laterally to insert along the arcus tendineus.
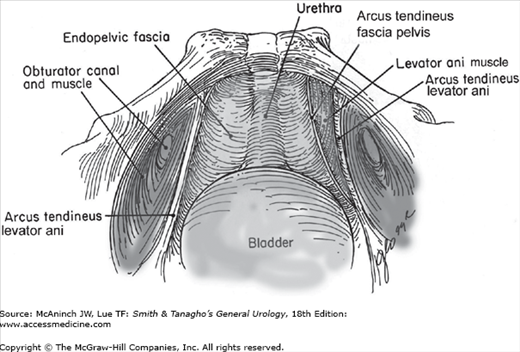
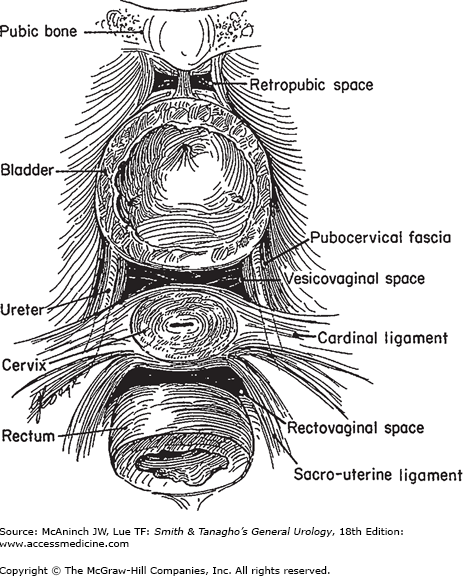
There are three important components of the pelvic fasciae. Anteriorly, the pubourethral ligaments attach to the lower portion of the pubis and insert on the proximal third of the urethra. Laterally, the arcus tendineus fascia pelvis extends from the pubourethral ligament (inferior portion of pubic symphysis) to the ischial spine. The arcus tendineus fascia pelvis is a thickening of the fascia overlying the iliococcygeus muscle. It corresponds to the lateral attachment of the anterior bladder wall to the pelvic sidewall (Figure 40–3). Fascia extending medially from this arc carries a variety of names (pubourethral, pubocervical, urethropelvic, vesicopelvic) and provides important support to the urethra and anterior vaginal wall. Posterior to the ischial spine, the fascia fans out to either side of the rectum and attaches to the pelvic sidewall as the strong cardinal and uterosacral ligaments (Figure 40–4). The cardinal and uterosacral ligaments hold the uterus and upper vagina in their proper place over the levator plate.
Many anatomic and surgical texts suggest that the levator ani muscles are dually innervated from the pudendal nerve on the perineal surface and direct branches of the sacral nerves on the pelvic surface. However, recent anatomic, neurophysiologic, and experimental evidence indicates that the levator ani muscles are innervated solely by a nerve traveling on the intrapelvic surface of the muscles without contribution of the pudendal nerve. This nerve originates from S3, S4, and/or S5 and innervates both the coccygeus and the levator ani muscle complex. After exiting the sacral foramina, it travels 2–3 cm medial to the ischial spine and ATLA across the coccygeus, iliococcygeus, pubococcygeus, and puborectalis. Given its location, the levator ani nerve is susceptible to injury during parturition and pelvic surgery.
The pudendal nerve innervates the striated urethral and anal sphincters as well as the deep and superficial perineal muscles and provides sensory innervation to the external genitalia. This nerve follows a complex course that originates from S2–S4 and travels behind the sacrospinous ligament just medial to the ischial spine, exiting the pelvis through the greater sciatic foramen. It then enters the ischiorectal fossa through the lesser sciatic foramen and travels through the pudendal canal (Alcock’s canal) on the medial aspect of the obturator internus muscles before separating into several terminal branches that terminate within the muscles and skin of the perineum.
Pathophysiology
Pelvic prolapse is prevented by several mechanisms. The most important support is from the continuous contraction of the levator ani pelvic muscles. The activity of skeletal muscle is a combination of basic tone, reflex contraction or relaxation, and voluntary contraction or relaxation. In patients with multiple deliveries, there is widening and descent of the levator plate. Therefore, the musculature becomes less important, and the “fascial” structures become the more important elements of support as the organs cross the pelvic floor.
Because of the complexity of pelvic organ support, vaginal prolapse is likely multifactorial: myopathic or neuropathic disorders, aging, atrophy, chronic increase in abdominal pressures, multiple deliveries, hysterectomy, and hormonal changes. However, intrinsic collagen abnormalities and other individual predisposing factors, such as genetics, differences in pelvic architecture, inherent quality of the pelvic musculature, and tissue response to injury, might explain why patients with known risk factors do not develop prolapse and many patients without risk factors do.
Classification
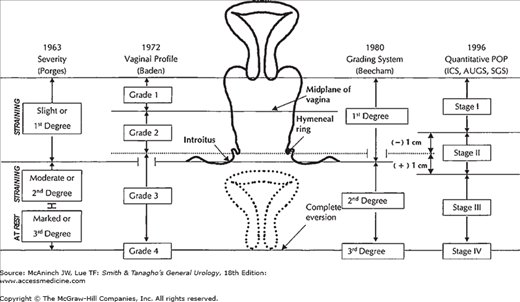
A number of classification systems have been published to facilitate standardization of clinical findings and enable communication about patients. Until recently, none of the systems have been validated. In 1996, the International Continence Society accepted standardization of the terminology for prolapse, known as the POPQ system for pelvic organ prolapse quantification. Although most clinicians still use the Baden-Walker system (Grades 1–4), the POPQ system is the accepted standard for clinical studies and published data on prolapse (Figure 40–5).
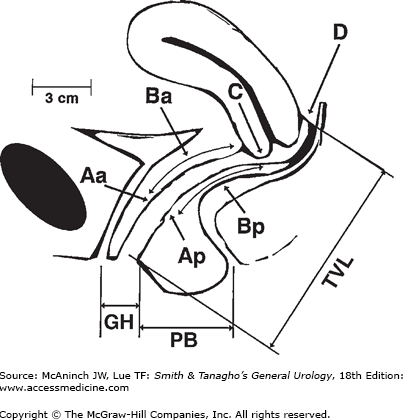
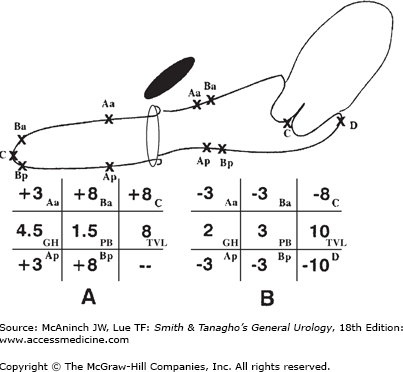
The POPQ system is a site-specific quantitative description of support that locates six defined points around the vagina (two anterior, two posterior, and two apical) with respect to their relationship to the hymenal ring. Negative numbers (in centimeters) are assigned to structures that have not prolapsed and positive numbers to those that protrude, with the plane of the hymen defined as zero. Points Aa and Ap are 3 cm above the hymenal ring. Points Ba and Bp are defined as the lowest points of the prolapse. The apex anteriorly is C (cervix), and posteriorly is D (pouch of Douglas). When the uterus is absent, point C is the vaginal cuff and D is omitted. TVL is total vaginal length at rest. GH is the genital hiatus measured from the middle of the urethral meatus to the posterior hymenal ring. PB is the perineal body measured from the posterior aspect of GH to the midanal opening (Figures 40–6 and 40–7).
Figure 40–6.
The pelvic organ prolapse quantification system. Aa, anterior vaginal wall 3 cm from hymen; Ba, lowest point of anterior vaginal wall prolapse; C, distance from hymen to cervix; D, distance from hymen to posterior fornix (pouch of Douglas); Ap, posterior vaginal wall 3 cm from hymen. Bp, lowest point of posterior vaginal wall prolapse; TVL, total vaginal length; GH, genital hiatus measured from midurethral meatus to posterior hymen; PB, perineal body measured from posterior hymen to midanus. (Reproduced, with permission, from Bump RC et al: The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10.)
Figure 40–7.
A: Example of complete vaginal prolapse (eversion) using POPQ classification. This occurs after a hysterectomy; therefore, there is no point D. Points Aa and Ap are maximally distal. Points Ba, C, and Bp are maximally everted. B: Normal support with no vaginal wall descent. (Reproduced, with permission, from Bump RC et al: The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 1996;175:10.)
The terminology avoids assigning a specific label, such as cystocele or rectocele, to the prolapsing part of the vagina, acknowledging that the actual organ(s) frequently cannot be determined on physical examination. Although it is more difficult to learn than traditional systems, reproducibility studies document interobserver and intraobserver reliability.
Diagnosis
Pelvic organ prolapse is often asymptomatic until it is severe. Many women present with symptoms in addition to the vaginal bulge, as a result of the associated organ dysfunction.
The most common complaint due to anterior compartment prolapse (cystocele) is vaginal bulging, with or without suprapubic pressure and pain. Other symptoms include urgency, frequency, urge incontinence, and recurrent urinary tract infections. Obstructive voiding symptoms are due to urethral kinking when the bladder descends beyond the pubic ramus, but the urethra remains fixed. This is commonly seen in the setting with previous surgery (eg, bladder neck suspension, urethropexy, sling). Patients may describe using unusual positions to void, such as pelvic tilting, squatting, or standing. Acute urinary retention and hydronephrosis are rare in patients with pelvic prolapse.
Many women with severe prolapse report that their stress incontinence improved as the prolapse worsened. Reduction of the prolapse during examination can produce stress incontinence in more than 50% of clinically continent patients. This unmasking of occult stress incontinence warrants attention when considering surgical therapy.
Constipation and difficult defecation with distal stool trapping or excessive straining are symptoms commonly attributed to posterior compartment prolapse (rectocele). The patient may report having to manually splint the perineum or vagina to assist evacuation.
A thorough physical examination should be done with a comfortably full bladder, at rest and with straining, in the supine (lithotomy) position. Depending on the patient’s ability to strain, examination in the upright position may be performed to accentuate the degree of prolapse to match that reported by the patient. The goal of examination is to determine the degree of prolapse, the specific anatomic defects, and the presence of concomitant organ prolapse in other compartments or incontinence. In the supine position, the origin of prolapse should be determined. Using a half-speculum blade, the posterior vaginal wall is retracted; the patient is asked to strain while the anterior defect is evaluated. After characterizing the anatomic defects, an attempt can be made to reduce the cystocele in order to elicit occult stress urinary incontinence and hypermobility. Similarly, the anterior vaginal wall is retracted to determine the presence of any posterior defect. A digital rectal exam assesses rectal tone, the presence of impacted stool, attenuation of the prerectal fascia, and perineal laxity. Using both blades of speculum, the vaginal vault or cervix can be examined for uterine descent, vault prolapse, and enterocele.
Evaluation
The basic evaluation for prolapse includes a history, physical examination, measurement of postvoid residual volume, and urinalysis. Additional testing may be needed in the setting of a large introital bulge where it may be difficult to differentiate between a severe cystocele, an enterocele, or high rectocele by physical examination only. Imaging studies can be performed to identify which organs are prolapsing. An ideal study should provide precise information about which structures are prolapsed, the presence of urinary retention and obstruction, urethral hypermobility, and urinary incontinence.
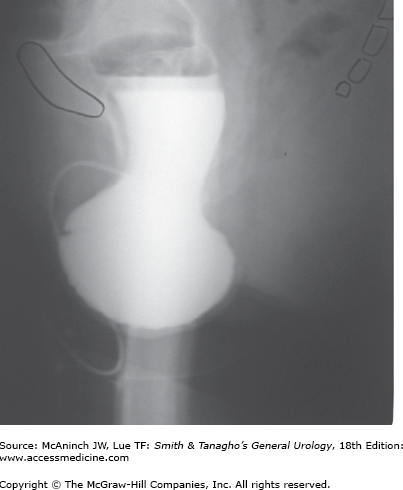
The patient is upright with a full bladder. Films are taken in a lateral position during both rest and strain. This exam provides information about bladder position, bladder neck funneling, urethral mobility, stress incontinence, and postvoid residual. The normal position of the base of the bladder should be above the pubococcygeal line (Figure 40–8). The presence of a rectocele can also be inferred when bowel gas is identified below the pubic symphysis. This exam is static and does not provide information about other pelvic organs or soft tissues of the pelvic floor.
Ultrasound is an attractive imaging modality because it is easy to perform, minimally invasive, and avoids radiation exposure. Tubo-ovarian and renal disease can also be assessed during examination. There is evidence that ultrasound is useful in evaluating bladder neck hypermobility; however, transvaginal imaging for pelvic prolapse does not provide adequate visualization of the soft tissues. Translabial ultrasound can be used to quantify prolapse, although it appears to be better for the anterior compartment and uterine descent than for the posterior compartment.
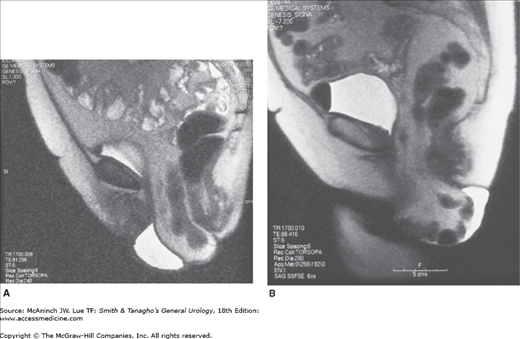
Recently, magnetic resonance imaging (MRI) has been used to evaluate pelvic prolapse. It is performed quickly, without contrast or ionizing radiation, and permits visualization of the soft tissues as well as the upper urinary tract. Gousse and colleagues (2000) have shown that in comparison with findings at surgery, MRI has a 100% sensitivity, 83% specificity, and a positive predictive value of 97% when assessing cystoceles. MRI does not require intravesical catheterization, does not require contrast, is fast, and is noninvasive. The drawback is that it may underestimate the extent of cystocele and enterocele because the exam must be performed in the supine position, which diminishes the downward forces that can be generated with abdominal straining. Standing MRIs will be the ultimate modality in obtaining an even more precise study for prolapse. Cost is the main reason that prohibits the wide use of MRIs currently (Figures 40–9A and B).
A urodynamic study can be done with or without video assistance. Both methods provide information about bladder compliance, capacity, sensation of filling, detrusor overactivity, and contractility. An advantage in performing videourodynamics is the capability to watch the position and funneling of the bladder neck during filling and straining. This combines cystourethrography with the urodynamic study. Choosing to use fluoroscopy should be based on cost, availability, and familiarity with this method.
The importance of documenting the presence of urinary incontinence in patients with large cystoceles is controversial. The incidence of occult stress urinary incontinence is felt to be as high as 22–80% among patients with high-stage vaginal prolapse. Due to the known masked urinary incontinence, and the high incidence of postoperative “de novo” stress incontinence, many pelvic reconstruction surgeons routinely perform a concomitant anti-incontinence surgery in all anterior vaginal reconstruction, independent of the continence status. This, however, remains a point of debate.
Determining the presence of bladder overactivity is important in preoperative counseling because it can affect the postoperative result. In the majority of cases (60–80%), urgency may resolve after surgery. However, some patients may have no change in urgency or a worsening of their urgency with sling placement and/or bladder neck elevation. Urodynamic study may also suggest urinary obstruction with elevated voiding pressure, low urinary flow, or radiographic evidence of urethral kinking.
This exam is performed to rule out concurrent pathology in the bladder and urethra, such as bladder carcinoma, urethral diverticulum, stones, or foreign bodies (ie, suture material) from previous surgery. Cystoscopic illumination can also be used to differentiate an enterocele from a cystocele. A pelvic exam is performed with the cystoscope in the bladder. The bladder transilluminates through the anterior vaginal wall so that the extent of the bladder prolapse is demarcated. It can also be used as a bedside urodynamic exam, assessing filling sensation, postvoid residual, and visual cystometrics for bladder contractions. With a full bladder, a supine Valsalva stress test can be performed, looking for urethral leakage.
Patients with a high-stage cystocele should have upper-tract imaging because there is a 4–7% incidence of moderate hydroureteronephrosis amongst patients with severe vaginal prolapse. This risk is greater in patients with procidentia, compared with vault prolapse. Ultrasound, excretory urography, computed tomography, or MRI can be used. An advantage of MRI is the ability to simultaneously evaluate the upper urinary tract, tubo-ovarian disease, and pelvic organ prolapse simultaneously.
Patients must have sterile urine prior to proceeding with an operative procedure. In preparation for surgery, a complete blood count, basic metabolic panel, coagulation profile, and urine culture should be obtained.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree