CHAPTER 17 Fecal Incontinence
Fecal incontinence is usually defined as the involuntary passage of fecal matter through the anus or the inability to control the discharge of bowel contents. The severity of incontinence can range from occasional unintentional elimination of flatus to the seepage of liquid fecal matter or the complete evacuation of bowel contents. Consequently, the problem has been difficult to characterize from an epidemiologic and pathophysiologic standpoint, but undoubtedly causes considerable embarrassment, a loss of self-esteem, social isolation, and a diminished quality of life.1
EPIDEMIOLOGY
Fecal incontinence affects people of all ages, but its prevalence is disproportionately higher in middle-aged women, older adults, and nursing home residents. Estimates of the prevalence of fecal incontinence vary greatly and depend on the clinical setting, definition of incontinence, frequency of occurrence, and influence of social stigma and other factors.2 Both the embarrassment and social stigma attached to fecal incontinence make it difficult for subjects to seek health care; consequently, treatment is often delayed for several years. Fecal incontinence not only causes significant morbidity in the community, but also consumes substantial health care resources.
In a U.S. householder survey, frequent leakage of stool or fecal staining for more than one month were reported by 7.1% and 0.7% of the population, respectively.3 In the United Kingdom, two or more episodes of fecal incontinence per month were reported by 0.8% of patients who presented to a primary care clinic.4 In an older self-caring population (older than 65 years), fecal incontinence occurred at least once a week in 3.7% of subjects and in more men than women (ratio of 1.5 : 1).5 The frequency of fecal incontinence increases with age, from 7% in women younger than 30 years to 22% in women in their seventh decade.6,7 By contrast, 25% to 35% of institutionalized patients and 10% to 25% of hospitalized geriatric patients have fecal incontinence.1 In the United States, fecal incontinence is the second leading reason for placement in a nursing home.
In a survey of 2570 households, comprising 6959 individuals, the frequency of at least one episode of fecal incontinence during the previous year was 2.2%; among affected persons, 63% were women, 30% were older than 65 years, 36% were incontinent of solid stool, 54% were incontinent of liquid stool, and 60% were incontinent of flatus.1 Furthermore, in another prospective survey of patients who attended either a gastroenterology or a primary care clinic, over 18% reported fecal incontinence at least once a week.8 Only one third had ever discussed the problem with a physician. When stratified for the frequency of episodes, 2.7% of patients reported incontinence daily, 4.5% weekly, and 7.1% monthly.8 In another survey, fecal incontinence was associated with urinary incontinence in 26% of women who attended a urology-gynecology clinic.9 A high frequency of mixed fecal and urinary incontinence was also reported in nursing home residents. Persons with incontinence were 6.8 times as likely to miss work or school, and missed an average of 50 work or school days per year, compared with those without incontinence or other functional gastrointestinal symptoms.3
HEALTH CARE BURDEN
The cost of health care related to fecal incontinence includes measurable components such as the evaluation, diagnostic testing, and treatment of incontinence, the use of disposable pads and other ancillary devices, skin care, and nursing care. Approximately $400 million/year is spent on adult diapers,8 and between $1.5 and $7 billion/year is spent on care for incontinence among institutionalized older patients.1,2,10 In a long-term facility, the annual cost for a patient with mixed fecal and urinary incontinence was $9,711.11 In the outpatient setting, the average cost per patient (including evaluation) has been estimated to be $17,166.12 In addition, these persons incur costs that cannot be easily measured and that result from their impaired quality of life and social dysfunction.7 Fecal incontinence is most likely to affect a person’s quality of life significantly and lead to increased use of health care, predominantly in women with moderate to severe symptoms.
PATHOPHYSIOLOGY
FUNCTIONAL ANATOMY AND PHYSIOLOGY OF THE ANORECTUM
A structurally and functionally intact anorectal unit is essential for maintaining normal continence of bowel contents (see Chapters 98 and 125).13 The rectum is a hollow muscular tube composed of a continuous layer of longitudinal muscle that interlaces with the underlying circular muscle. This unique muscle arrangement enables the rectum to serve both as a reservoir for stool and as a pump for emptying stool. The anus is a muscular tube 2 to 4 cm in length that at rest forms an angle with the axis of the rectum (Fig. 17-1). At rest, the anorectal angle is approximately 90 degrees; with voluntary squeeze, the angle becomes more acute, approximately 70 degrees; and during defecation the angle becomes obtuse, about 110 to 130 degrees.
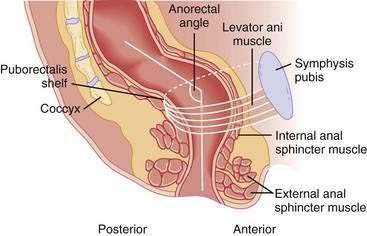
Figure 17-1. Sagittal diagrammatic view of the anorectum.
(From Rao SSC. Pathophysiology of adult fecal incontinence. Gastroenterology 2004; 126:S14-22.)
The anal sphincter consists of two muscular components—the internal anal sphincter (IAS), a 0.3- to 0.5-cm thick expansion of the circular smooth muscle layer of the rectum, and the external anal sphincter (EAS), a 0.6- to 1.0-cm thick expansion of the levator ani muscles. Morphologically, both sphincters are separate and heterogenous.14 The IAS is composed predominantly of slow-twitch, fatigue-resistant smooth muscle and generates mechanical activity with a frequency of 15 to 35 cycles/min as well as ultraslow waves at 1.5 to 3 cycles/min.13 The IAS contributes approximately 70% to 85% of the resting anal sphincter pressure but only 40% of the pressure after sudden distention of the rectum and 65% during constant rectal distention; the remainder of the pressure is provided by the EAS.15 Therefore, the IAS is responsible chiefly for maintaining anal continence at rest.
The anus is normally closed by the tonic activity of the IAS. This barrier is reinforced during voluntary squeeze by the EAS. The anal mucosal folds, together with the expansive anal vascular cushions (see later), provide a tight seal.16 These barriers are augmented by the puborectalis muscle, which forms a flap-like valve that creates a forward pull and reinforces the anorectal angle.13
The anorectum is richly innervated by sensory, motor, and autonomic nerves and by the enteric nervous system. The principal nerve to the anorectum is the pudendal nerve, which arises from the second, third, and fourth sacral nerves (S2, S3, S4), innervates the EAS, and subserves sensory and motor function.17 A pudendal nerve block creates a loss of sensation in the perianal and genital skin and weakness of the anal sphincter muscle but does not affect rectal sensation.15 A pudendal nerve block also abolishes the rectoanal contractile reflexes (see later), an observation that suggests that pudendal neuropathy may affect the rectoanal contractile reflex response. The sensation of rectal distention is most likely transmitted along the S2, S3, and S4 parasympathetic nerves. These nerve fibers travel along the pelvic splanchnic nerves and are independent of the pudendal nerve.13
How humans perceive stool contents in the anorectum is not completely understood. Earlier studies failed to demonstrate rectal sensory awareness.13 Subsequent studies have confirmed that balloon distention is perceived in the rectum and that such perception plays a role in maintaining continence.16,18 Furthermore, sensory conditioning can improve hyposensitivity19,20 and hypersensitivity21 of the rectum. Mechanical stimulation of the rectum can produce cerebral evoked responses,22 thereby confirming that the rectum is a sensory organ.
Although organized nerve endings are not present in the rectal mucosa or myenteric plexus, myelinated and unmyelinated nerve fibers are present.13 These nerves most likely mediate the distention or stretch-induced sensory responses as well as the viscerovisceral,22 rectoanal inhibitory, and rectoanal contractile reflexes. The sensation of rectal distention is most likely transmitted via the parasympathetic nervi erigentes along the S2, S3, and S4 splanchnic nerves. Rectal sensation and the ability to defecate can be abolished completely by resection of the nervi erigentes.23 If parasympathetic innervation is absent, rectal filling is perceived only as a vague sensation of discomfort. Even persons with paraplegia or sacral neuronal lesions may retain some degree of sensory function, but almost no sensation is felt if lesions occur in the higher spine.15,18,24 Therefore, the sacral nerves are intimately involved in the maintenance of continence.
The suggestion has been made that bowel contents are sensed periodically by anorectal sampling,25 the process whereby transient relaxation of the IAS allows the stool contents from the rectum to come into contact with specialized sensory organs in the upper anal canal. Specialized afferent nerves may exist that subserve sensations of touch, temperature, tension, and friction, but the mechanisms are incompletely understood.13 Incontinent patients appear to sample rectal contents less frequently than continent subjects. The likely role of anal sensation is to facilitate discrimination between flatus and feces and the fine-tuning of the continence barrier, but its precise role has not been well characterized. Rectal distention is associated with a fall in anal resting pressure known as the rectoanal inhibitory reflex. The amplitude and duration of this relaxation increases with the volume of rectal distention. This reflex is mediated by the myenteric plexus and is present in patients in whom the hypogastric nerves have been transected and in those with a spinal cord lesion. The reflex is absent after transection of the rectum, but it may recover.18 Although the rectoanal inhibitory reflex may facilitate discharge of flatus, rectal distention is also associated with a rectoanal contractile response, a subconscious reflex effort to prevent release of rectal contents, such as flatus.26,27 This contractile response involves contraction of the EAS and is mediated by the pelvic splanchnic and pudendal nerves. The amplitude and duration of the rectoanal contractile reflex also increases with rectal distention, up to a maximum volume of 30 mL. Abrupt increases in intra-abdominal pressure, as caused by coughing or laughing, are associated with an increase in anal sphincter pressure. A number of mechanisms, including reflex contraction of the puborectalis, may be involved.
The blood-filled vascular tissue of the anal mucosa also plays an important role in producing optimal closure of the anus. An in vitro study has shown that even during maximal involuntary contraction, the internal sphincter ring is unable to close the anal orifice completely, and a gap of approximately 7 mm remains. This gap is filled by the anal cushions, which may exert pressures of up to 9 mm Hg and thereby contribute 10% to 20% to the resting anal pressure.26
PATHOGENIC MECHANISMS
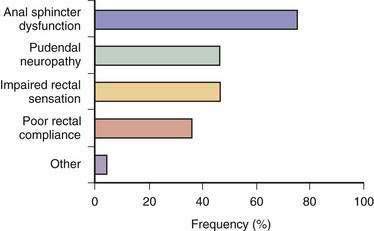
Fecal incontinence occurs when one or more mechanisms that maintain continence is disrupted to the extent that other mechanisms are unable to compensate. Therefore, fecal incontinence is often multifactorial.2,27 In a prospective study, 80% of patients with fecal incontinence had more than one pathogenic abnormality (Fig. 17-2).13 Although the pathophysiologic mechanisms often overlap, they can be categorized under four broad groups, as summarized in Table 17-1.
Table 17-1 Mechanisms, Causes, and Pathophysiology of Fecal Incontinence
| MECHANISM | CAUSES | PATHOPHYSIOLOGY |
|---|---|---|
| Abnormal Anorectal or Pelvic Floor Structures | ||
| Anal sphincter muscle | Hemorrhoidectomy, neuropathy, obstetric injury | Sphincter weakness, loss of sampling reflex |
| Puborectalis muscle | Aging, excessive perineal descent, trauma | Obtuse anorectal angle, sphincter weakness |
| Pudendal nerve | Excessive straining, obstetric or surgical injury, perineal descent | Sphincter weakness, sensory loss, impaired reflexes |
| Nervous system, spinal cord, autonomic nervous system | Avulsion injury, spine surgery, diabetes mellitus, head injury, multiple sclerosis, spinal cord injury, stroke | Loss of sensation, impaired reflexes, secondary myopathy, loss of accommodation |
| Rectum | Aging, inflammatory bowel disease, irritable bowel syndrome, prolapse, radiation | Loss of accommodation, loss of sensation, hypersensitivity |
| Abnormal Anorectal or Pelvic Floor Function | ||
| Impaired anorectal sensation | Autonomic nervous system disorders, central nervous system disorders, obstetric injury | Loss of stool awareness, rectoanal agnosia |
| Fecal impaction | Dyssynergic defecation | Fecal retention with overflow, impaired sensation |
| Altered Stool Characteristics | ||
| Increased volume and loose consistency | Drugs, bile salt malabsorption, infection, inflammatory bowel disease, irritable bowel syndrome, laxatives, metabolic disorders | Diarrhea and urgency, rapid stool transport, impaired accommodation |
| Hard stools, retention | Drugs, dyssynergia | Fecal retention with overflow |
| Miscellaneous | ||
| Physical mobility, cognitive function | Aging, dementia, disability | Multifactorial changes |
| Psychosis | Willful soiling | Multifactorial changes |
| Drugs* | ||
| Food intolerance | Fructose, lactose, or sorbitol malabsorption | Diarrhea, flatus |
* Pathophysiology is noted for each class of drugs.
Abnormal Anorectal and Pelvic Floor Structures
Anal Sphincter Muscles
Disruption or weakness of the EAS muscle causes urge-related or diarrhea-associated fecal incontinence. In contrast, damage to the IAS muscle or anal endovascular cushions may lead to a poor seal and an impaired sampling reflex. These changes may cause passive incontinence or fecal seepage (see later), often under resting conditions. Both sphincters may be defective in many patients. The extent of muscle loss can influence the severity of incontinence.13
The most common cause of anal sphincter disruption is obstetric trauma, which may involve the EAS, IAS, or pudendal nerves. Why most women who have sustained an obstetric injury in their 20s or 30s typically do not present with fecal incontinence until their 50s, however, is unclear. In a prospective study, 35% of primiparous (normal antepartum) women showed evidence of anal sphincter disruption after vaginal delivery.28,29 Other important risk factors include a forceps-assisted delivery, prolonged second stage of labor, large birth weight, and occipitoposterior presentation.13 A prospective study of 921 primiparous women has shown that the frequencies of fecal incontinence at 6 weeks and 6 months postpartum are 27% and 17%, respectively, in subjects with vaginal delivery and a sphincter tear; 11% and 8%, respectively, in subjects with vaginal delivery but without a tear; and 10% and 7.6%, respectively, in subjects who underwent cesarean section.30 This study showed clearly that the occurrence and severity of fecal incontinence were attributable to an anal sphincter tear that occurred at the time of vaginal delivery.
Episiotomy is believed to be a risk factor for anal sphincter disruption. In one study, medial episiotomy was associated with a ninefold higher risk of anal sphincter dysfunction.31 Regardless of the type of delivery, however, incontinence of feces or flatus occurred in a surprisingly large percentage of middle-aged women, thereby suggesting that age-related changes in the pelvic floor may predispose to fecal incontinence.
Aging affects anal sphincter function.32 In men and women older than 70 years, sphincter pressures decrease by 30% to 40% compared with younger persons.33 Also, in all age groups, anal squeeze pressure is lower in women than men,33 with a rapid fall after menopause.34 Estrogen receptors have been identified in the human striated anal sphincter, and ovariectomy in rats leads to atrophy of the striated anal sphincter muscle.13,35 These observations suggest that the strength and vigor of the pelvic floor muscles are influenced by hormones. Pudendal nerve terminal motor latency (PNTML) is prolonged in older women, and pelvic floor descent is excessive on straining.36 These mechanisms may contribute to progressive damage to the striated anal sphincter muscle. Aging is also associated with increased thickness and echogenicity of the IAS.37
Other causes of anatomic disruption include anorectal surgery for hemorrhoids, fistulas, and fissures. Anal dilation or lateral sphincterotomy may result in incontinence because of fragmentation of the anal sphincters.38 Hemorrhoidectomy can cause incontinence by inadvertent damage to the IAS39 or loss of endovascular cushions. Accidental perineal trauma or a pelvic fracture may also cause direct sphincter trauma that leads to fecal incontinence,40 but anoreceptive intercourse is not associated with anal sphincter dysfunction.41 Finally, IAS dysfunction may also occur because of myopathy, degeneration, or radiotherapy.13
Puborectalis Muscle
The puborectalis muscle is also important for maintaining continence by forming a flap valve mechanism.42 Studies using three-dimensional ultrasound have shown that 40% of women with fecal incontinence have major abnormalities, and another 32% have minor abnormalities of the puborectalis muscle, compared with 21% and 32%, respectively, of asymptomatic parous controls.43 Also, assessment of puborectalis function by a perineal dynamometer revealed impaired puborectalis (levator ani) contraction in patients with fecal incontinence, and this finding was an independent risk factor for and correlated with the severity of fecal incontinence.44 Furthermore, improvement in puborectalis strength following biofeedback therapy was associated with clinical improvement, in part because the upper portion of the puborectalis muscle receives its innervations from branches of the S3 and S4 sacral nerves rather than the pudendal nerve. Therefore, the puborectalis muscle and EAS have separate neurologic innervations. Consequently, pudendal blockage does not abolish voluntary contraction of the pelvic floor45 but completely abolishes EAS function.15
Nervous System
Intact innervation of the pelvic floor is essential for maintaining continence. Sphincter degeneration secondary to pudendal neuropathy and obstetric trauma may cause fecal incontinence in women.28 The neuropathic injury is often sustained during childbirth, probably as a result of stretching of the nerves during elongation of the birth canal or direct trauma during the passage of the fetal head. The nerve damage is more likely to occur when the fetal head is large, the second stage of labor is prolonged, or forceps are applied, especially with a high-forceps delivery or prolonged labor.
The role of extrinsic autonomic innervation is somewhat controversial. Animal studies have shown that the pelvic nerves convey fibers that relax the rectum.46 Consequently, these nerves may play a role in accommodating and storing feces and gas. Damage to the pelvic nerves may lead to impaired accommodation and rapid transit through the rectosigmoid region, thereby overwhelming the continence barrier mechanisms. Sympathetic efferent activity, as studied by stimulating the presacral sympathetic nerves, tends to relax the IAS, whereas parasympathetic stimulation may cause contraction of the anal sphincter. The upper motor neurons for voluntary sphincter muscle lie close to those that innervate the lower limb muscles in the parasagittal motor cortex, adjacent to the sensory representation of the genitalia and perineum in the sensory cortex.13 Consequently, damage to the motor cortex from a central nervous system (CNS) lesion may lead to incontinence. In some patients with neurogenic incontinence, the sensory and motor nerve fibers may be damaged, resulting in sensory impairment.47 This damage can impair conscious awareness of rectal filling as well as the associated reflex responses in the striated pelvic floor sphincter muscles.
Approximately 10% of patients with fecal incontinence may have a lesion more proximal than the intrapelvic or perianal nerves. The primary abnormality in these patients is cauda equina nerve injury,48 which may be occult and not evident through clinical evaluation. These patients have a prolongation of nerve conduction along the cauda equina nerve roots without an abnormality in PNTML.49 In a minority of patients, however, a combination of peripheral and central lesions is present. Other disorders such as multiple sclerosis, diabetes mellitus, and demyelination injury (or toxic neuropathy from alcohol or traumatic neuropathy) may also lead to incontinence.13
Rectum
The rectum is a compliant reservoir that stores stool until social conditions are conducive to its evacuation.2 If rectal wall compliance is impaired, a small volume of stool material can generate a high intrarectal pressure that can overwhelm anal resistance and cause incontinence.50 Causes include radiation proctitis, ulcerative colitis, or Crohn’s disease, infiltration of the rectum by tumor, and radical hysterectomy.51 Similarly, rectal surgery, particularly pouch surgery,52 and spinal cord injury53 may be associated with loss of rectal compliance.
Abnormal Anorectal and Pelvic Floor Function
Impaired Anorectal Sensation
An intact sensation not only provides a warning of imminent defecation, but also helps distinguish among formed stool, liquid feces, and flatus. Older persons,54 those who are physically and mentally challenged, and children with fecal incontinence55 often show blunted rectal sensation. Impaired rectal sensation may lead to excessive accumulation of stool, thereby causing fecal impaction, megarectum (extreme dilatation of the rectum), and fecal overflow. Causes of impaired sensation include neurologic damage such as multiple sclerosis, diabetes mellitus, and spinal cord injury.53 Less well known is that analgesics (particularly opiates) and antidepressants also may impair rectal sensation and produce fecal incontinence. The importance of the rectum in preserving continence has been demonstrated conclusively through surgical studies in which preservation of the distal 6 to 8 cm of the rectum, along with its parasympathetic nerve supply, helped subjects avoid incontinence.56 By contrast, rectal sensation and the ability to defecate can be abolished completely by resection of the nervi erigentes (see earlier).23
An intact sampling reflex allows an individual to choose whether to discharge or retain rectal contents. Conversely, an impaired sampling reflex may predispose a subject to incontinence.25 The role of the sampling reflex in maintaining continence, however, remains unclear. In children who have undergone colonic pull-through surgery (see Chapter 113), some degree of sensory discrimination is preserved.57 Because the anal mucosal sensory zone is absent in these children, the suggestion has been made that sensory receptors, possibly located in the puborectalis muscle, may play a role in facilitating sensory discrimination. Also, traction on the muscle is a more potent stimulus for triggering defecation and a sensation of rectal distention. Because abolition of anal sensation by the topical application of 5% lidocaine does not reduce resting sphincter pressure (although it affects voluntary squeeze pressure but does not affect the ability to retain saline infused into the rectum), the role of anal sensation in maintaining fecal continence has been questioned.13
Dyssynergic Defecation and Incomplete Stool Evacuation
In some patients, particularly older adults, prolonged retention of stool in the rectum or incomplete evacuation may lead to seepage of stool or staining of undergarments.54 Most of these patients show obstructive or dyssynergic defecation,58 and many of them also exhibit impaired rectal sensation, whereby anal sphincter and pudendal nerve function is intact but the ability to evacuate a simulated stool is impaired. Similarly, in older adults and in children with functional incontinence, the prolonged retention of stool in the rectum can lead to fecal impaction. Fecal impaction may also cause prolonged relaxation of the IAS, thereby allowing liquid stool to flow around impacted stool and to escape through the anal canal (see Chapter 18).55
Descending Perineum Syndrome
In women with long-standing constipation and a history of excessive straining for many years (perhaps even without prior childbirth), excessive straining may lead to progressive denervation of the pelvic floor muscles.59 Most of these patients demonstrate excessive perineal descent and sphincter weakness, which may lead to rectal prolapse; however, fecal incontinence is not an inevitable consequence. Whether or not incontinence develops will depend on the state of the pelvic floor and the strength of the sphincter muscles.
Altered Stool Characteristics
The consistency, volume, and frequency of stool and the presence or absence of irritants in stool also may play a role in the pathogenesis of fecal incontinence.2 In the presence of large-volume liquid stools, which often transit the hindgut rapidly, continence can only be maintained through intact sensation and a strong sphincteric barrier. Similarly, in patients with bile salt malabsorption, lactose or fructose intolerance, or rapid dumping of osmotic material into the colon, colonic transit of gaseous and stool contents is too rapid and can overwhelm the continence mechanisms (see Chapters 15 and 101).2
Miscellaneous Mechanisms
Various medical conditions and disabilities may predispose to fecal incontinence, particularly in older adults. Immobility and lack of access to toileting facilities are primary causes of fecal incontinence in this population.60 Several drugs may inhibit sphincter tone. Some are used to treat urinary incontinence and detrusor instability, including anticholinergics such as tolterodine tartarate (Detrol) and oxybutynin (Ditropan) and muscle relaxants such as baclofen (Lioresal), and cyclobenzaprine (Flexeril). Stimulants such as caffeinated products, fiber supplements, or laxatives may produce fecal incontinence by causing diarrhea.13
EVALUATION
CLINICAL FEATURES
The first step in the evaluation of a patient with fecal incontinence is to establish a trusting relationship with the patient and assess the duration and nature of the symptoms, with specific attention to whether the leakage consists of flatus, liquid stool, or solid stool and to the impact of the symptoms on the quality of the patient’s life (Table 17-2). Because many people misinterpret fecal incontinence as diarrhea or urgency,61 a detailed characterization of the symptom(s) is important. The clinician should ask about the use of pads or other devices and the patient’s ability to discriminate between formed or unformed stool and gas (the lack of such discrimination is termed rectal agnosia).2 An obstetric history; history of coexisting conditions such as diabetes mellitus, pelvic radiation, neurologic problems, or spinal cord injury; dietary history; and history of coexisting urinary incontinence are important. A prospective stool diary can be useful. The circumstances under which incontinence occurs should also be determined. Such a detailed inquiry may facilitate the recognition of the following types of fecal incontinence:
Table 17-2 Features of the History That Should Be Elicited from a Patient with Fecal Incontinence
Although overlap exists among these three types, by determining the predominant pattern, useful insights can be gained regarding the underlying mechanism(s) and preferred management. Symptom assessment, however, may not correlate well with manometric findings (see later). In one study, leakage had a sensitivity of 98.9%, specificity of 11%, and positive predictive value of 51% for detecting a low resting anal sphincter pressure on manometry.62 The positive predictive value for detecting a low anal squeeze pressure was 80%. Therefore, for an individual patient with fecal incontinence, the history and clinical features alone are insufficient to define the pathophysiology, and objective testing is essential63,64 (see later).
On the basis of the clinical features, several grading systems have been proposed. A modification of the Cleveland Clinic grading system65 has been validated by the St. Mark’s investigators66 and provides an objective method of quantifying the degree of incontinence. It can also be useful for assessing the efficacy of therapy. This grading system is based on seven parameters that include the following: (1-3) the character of the anal discharge as solid, liquid, or flatus; (4) the degree of alterations in lifestyle; (5, 6) the need to wear a pad or take antidiarrheal medication; and (7) the ability to defer defecation. The total score ranges from 0 (continent) to 24 (severe incontinence). As noted earlier, however, clinical features alone are insufficient to define the pathophysiology. The use of validated questionnaires such as the SCL-90R (Symptom Checklist-90-R) and SF-36 (Short-Form 36) surveys may provide additional information regarding psychosocial issues and the impact of fecal incontinence on the patient’s quality of life.
PHYSICAL EXAMINATION
A detailed physical examination, including a neurologic examination, should be performed in any patient with fecal incontinence, because incontinence may be secondary to a systemic or neurologic disorder. The focus of the examination is on the perineum and anorectum. Perineal inspection and digital rectal examination are best performed with the patient lying in the left lateral position and with good illumination. On inspection, the presence of fecal matter, prolapsed hemorrhoids, dermatitis, scars, skin excoriations, or a gaping anus and the absence of perianal creases may be noted. These features suggest sphincter weakness or chronic skin irritation and provide clues regarding the underlying cause.2 Excessive perineal descent or rectal prolapse can be demonstrated by asking the patient to attempt defecation. An outward bulge that exceeds 3 cm is usually defined as excessive perineal descent (see Chapter 18).67
Perianal sensation should be checked. The anocutaneous reflex examines the integrity of the connections between the sensory nerves and the skin; the intermediate neurons in spinal cord segments S2, S3, and S4; and the motor innervation of the external anal sphincter. This reflex can be assessed by gently stroking the perianal skin with a cotton bud in each perianal quadrant. The normal response consists of a brisk contraction of the external anal sphincter (“anal wink”). An impaired or absent anocutaneous reflex suggests either afferent or efferent neuronal injury.2
The accuracy of the digital rectal examination has been assessed in several studies. In one study of 66 patients, digital rectal examination by an experienced surgeon correlated somewhat with resting sphincter pressure (r = 0.56; P < 0.001) or maximum squeeze pressure (r = 0.72; P < 0.001).68 In a study of 280 patients with various anorectal disorders, a reasonable correlation was reported between digital examination and manometric findings, but the sensitivity, specificity, and positive predictive values of digital examination were low.69 In another study of 64 patients, the agreement between digital rectal examination and resting or squeeze pressure was 0.41 and 0.52, respectively.70 These data suggest that digital rectal examination provides only an approximation of sphincter strength. The findings are influenced by many factors, including the size of the examiner’s finger, technique used, and cooperation of the patient. One study has shown that trainees lack adequate skills for recognizing the features of fecal incontinence on digital rectal examination.71 Therefore, digital rectal examination is not reliable and is prone to interobserver differences. Digital rectal examination can identify patients with fecal impaction and overflow but is not accurate for diagnosing sphincter dysfunction and should not be used as the basis for decisions regarding treatment.2
DIAGNOSTIC TESTING
The first step in assessing a patient with fecal incontinence is to determine whether the incontinence is secondary to diarrhea or independent of stool consistency. If diarrhea coexists with incontinence, appropriate tests should be performed to identify the cause of the diarrhea (see Chapter 15). Such testing may include flexible sigmoidoscopy or colonoscopy to exclude colonic mucosal inflammation, a rectal mass, or stricture and stool studies for infection, volume, osmolality, electrolytes, fat content, and pancreatic function. Biochemical tests should be performed to look for thyroid dysfunction, diabetes mellitus, and other metabolic disorders. Breath tests may be considered for lactose or fructose intolerance or small intestinal bacterial overgrowth.2 A history of cholecystectomy may suggest bile salt malabsorption and prompt a therapeutic trial of a bile salt–binding agent.
Specific tests are available for defining the underlying mechanisms of fecal incontinence and are often used in complementary fashion. The most useful tests are anorectal manometry, anal endosonography, the balloon expulsion test, and PNTML.2,72–74
Anorectal Manometry and Sensory Testing
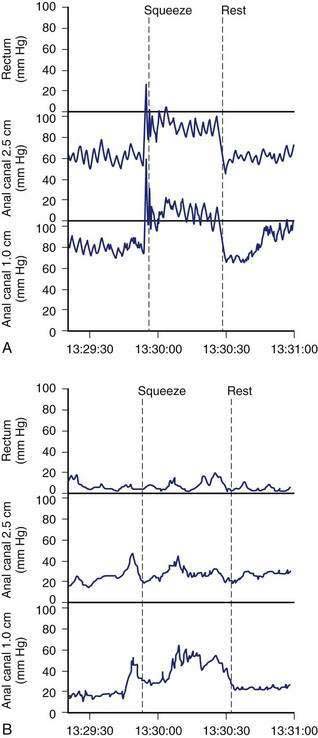
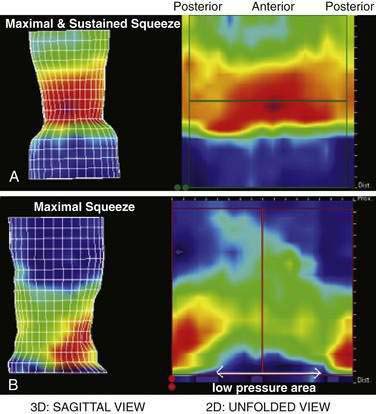
Anorectal manometry is a simple and useful method for assessing IAS and EAS pressures (Fig. 17-3) as well as rectal sensation, rectoanal reflexes, and rectal compliance. Several types of probes and pressure recording devices are available. Each system has distinct advantages and drawbacks. A water-perfused probe with multiple closely spaced sensors is commonly used.2 Increasingly, a solid-state probe with microtransducers or air-filled miniaturized balloons is used. A novel solid-state probe with 36 circumferential sensors spaced at 1-cm intervals, with a 4.2-mm outer diameter (Sierra Scientific Instruments, Los Angeles) has been reported to provide higher resolution than older style probes.75 This device uses a novel pressure transduction technology (TactArray) that allows each of the 36 pressure sensing elements to detect pressure over a length of 2.5 mm and in each of 12 radially dispersed sectors. The data can be displayed in isobaric contour plots that can provide a continuous dynamic representation of pressure changes, although anal sphincter pressures are higher than those recorded with water-perfused manometry. A high-definition manometry system with 256 circumferentially arrayed sensors in a 5-cm probe76 has become available and may provide anal sphincter pressure profiles and topographic changes of even higher fidelity (Fig. 17-4).
Anal sphincter pressures can be measured by stationary or station pull-through techniques.73,74 Resting anal sphincter pressure predominantly represents IAS function, and voluntary anal squeeze pressure predominantly represents EAS function. Patients with fecal incontinence have low resting and low squeeze pressures (see Figs. 17-3 and 17-4), indicating IAS and EAS weakness.2,69 The duration of sustained squeeze pressure provides an index of sphincter muscle fatigue. The ability of the EAS to contract reflexively can be assessed during abrupt increases in intra-abdominal pressure, as when the patient coughs. This reflex response causes the anal sphincter pressure to rise above that of the rectal pressure to preserve continence. The response may be triggered by receptors in the pelvic floor and mediated through a spinal reflex arc. In patients with a spinal cord lesion above the conus medullaris, this reflex response is preserved, even though voluntary squeeze may be absent, whereas in patients with a lesion of the cauda equina or sacral plexus, both the reflex and voluntary squeeze responses are absent.2,77,78
Rectal Sensory Testing
Rectal balloon distention with air or water can be used to assess sensory responses and compliance of the rectal wall. By distending a balloon in the rectum with incremental volumes, the thresholds for first perception, first desire to defecate, and urgent desire to defecate can be assessed. A higher threshold for sensory perception indicates reduced rectal sensitivity.2,77,79 The balloon volume required for partial or complete inhibition of anal sphincter tone also can be assessed. The volume required to induce reflex anal relaxation is lower in incontinent patients than in controls.80
Because sampling of rectal contents by the anal mucosa may play an important role in maintaining continence,25 quantitative assessment of anal perception using electrical or thermal stimulation has been advocated but is not used clinically.2 Rectal compliance can be calculated by assessing the changes in rectal pressure during balloon distention with air or fluid.73,81 Rectal compliance is reduced in patients with colitis,50 patients with a low spinal cord lesion, and diabetic patients with incontinence but is increased in those with a high spinal cord lesion.
Anorectal manometry can provide useful information regarding anorectal function.72,73,82 The American Motility Society has provided consensus guidelines and minimal standards for manometry testing.74 Although there are insufficient data regarding normal values, overlap betwen healthy subjects and patients with fecal incontinence,69 and large confidence intervals for test reproducibility,83 manometry testing can be useful for the individual patient with fecal incontinence.74 Manometric tests of anorectal function may also be useful for assessing objective improvement following drug therapy, biofeedback therapy, or surgery.84–86
Imaging the Anal Canal
Anal Endosonography
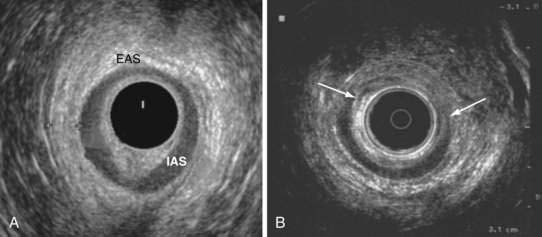
Anal endosonography is performed by using a 7- to 15-mHz rotating transducer with a focal length of 1 to 4 cm.87 The test provides an assessment of the thickness and structural integrity of the EAS and IAS and can detect scarring, loss of muscle tissue, and other local pathology (Fig. 17-5).88 Higher frequency (10- to 15-mHz) probes that provide better delineation of the sphincter complex have become available.88
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree