Esophagus
1.1 Iron pill esophagitis vs. Squamous dysplasia
1.2 Reactive multinucleated squamous cells vs. Herpes virus cytopathic effect
1.3 Reactive stromal changes vs. Cytomegalovirus esophagitis
1.4 Goblet cells in Barrett esophagus vs. Multilayered epithelium
1.5 Goblet cells of Barrett mucosa vs. Esophageal submucosal glands
1.6 Goblet cells of Barrett mucosa vs. Alcianophilic foveolar cells
1.7 Carryover of small bowel mucosa into esophageal sample vs. Barrett mucosa
1.8 Complete intestinal metaplasia vs. Incomplete intestinal metaplasia
1.9 Oncocytic change of submucosal glands vs. Pyloric gland adenoma
1.10 Eosinophilic esophagitis vs. Reflux esophagitis
1.11 Taxane effect vs. Squamous and columnar epithelial dysplasia
1.12 Esophagitis dissecans (sloughing esophagitis) vs. Esophageal pemphigus vulgaris
1.13 Lichenoid esophagitis vs. Reflux change
1.14 Atypical stromal cells in ulcers vs. Sarcomatoid squamous cell carcinoma
1.15 Multilayered epithelium vs. Squamous dysplasia
1.16 Columnar epithelial dysplasia vs. Reactive epithelial changes
1.17 Low-grade columnar epithelial dysplasia vs. High-grade columnar epithelial dysplasia
1.18 High-grade columnar epithelial dysplasia vs. Intramucosal adenocarcinoma
1.19 Lamina propria in columnar-lined esophagus vs. Submucosa in columnar-lined esophagus
1.20 Pseudoepitheliomatous hyperplasia vs. Squamous cell carcinoma
1.21 Esophageal smooth muscle tumors vs. Esophageal gastrointestinal stromal tumors
1.22 Melanoma vs. Esophageal gastrointestinal stromal tumor
1.1 Iron pill esophagitis vs. Squamous dysplasia
| Iron Pill Esophagitis | Squamous Dysplasia | |
|---|---|---|
| Age/Gender | No specific age; female predominance | Typically adults; male predominance |
| Location | Any part of esophagus; more likely in area of preexisting stricture | Often in middle third of the esophagus but can be anywhere |
| Symptoms | Dysphagia | No specific symptoms unless accompanied by invasive carcinoma (dysphagia) |
| Signs | None—patient may have iron deficiency anemia and thus taking iron supplements | No specific signs |
| Etiology | Damage due to mechanical effects of pill in preexisting ulcer/erosion as well as to toxicity of the medication itself | Like squamous cell carcinoma, associated with alcohol, smoking, male gender, ALDH1 polymorphisms |
| Histology | ||
| 1. Ulcer or erosion with golden brown pigment embedded within the lesion (early injury accompanied by regenerative squamous epithelium with reparative changes including enlarged nuclei and prominent nucleoli) (Fig. 1.1.1) 2. Pigment becomes more green-blue-black over time (Fig. 1.1.2) 3. Iron staining shows deeper pigment than apparent on H&E-stained slides (Fig. 1.1.3) | 1. Usually without ulcers; cells show nuclear hyperchromasia, lack nucleoli (Fig. 1.1.4) 2. Sometimes accompanied by invasive carcinoma (Fig. 1.1.5) 3. Not pigmented (Fig. 1.1.6) | |
| Special studies |
|
|
| Treatment | Iron pills can be administered with abundant fluids or with soft foods (applesauce, yogurt) | Ablation therapy such as radiofrequency ablation |
| Prognosis | Most symptoms resolve quickly | Low-grade dysplasia unlikely to progress to invasive carcinoma, whereas high-grade dysplasia has a high risk of progression |
Figure 1.1.1 Iron pill esophagitis. Note the brown appearance of the iron embedded in eroded squamous epithelium. At the bottom of the field, the tissue has a more purplish appearance attributable to oxidization.
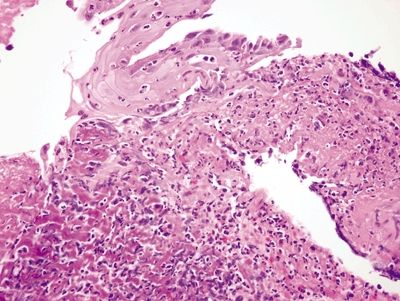
Figure 1.1.2 Iron pill esophagitis. In this image, the iron deposition is more subtle but has resulted in a purplish appearance of the damaged tissue in the left side of the field. Note the striking reactive squamous epithelial changes.
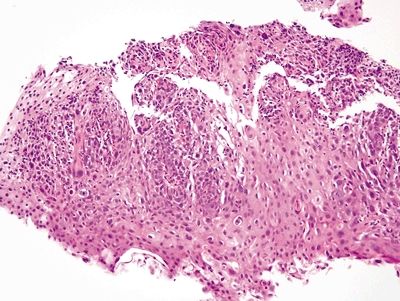
Figure 1.1.4 Squamous dysplasia. There is no erosion and no foreign debris. The nuclei, even at this magnification, are more hyperchromatic than those in the reparative squamous epithelium in Figure 1.1.2.
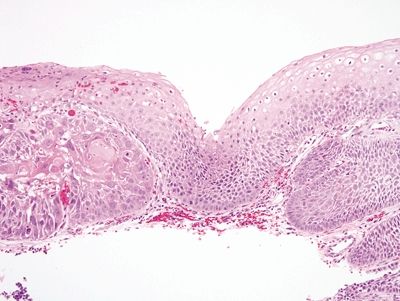
Figure 1.1.5 Squamous dysplasia. There is low-grade dysplasia on the right and an early carcinoma on the left in a background lacking inflammation.
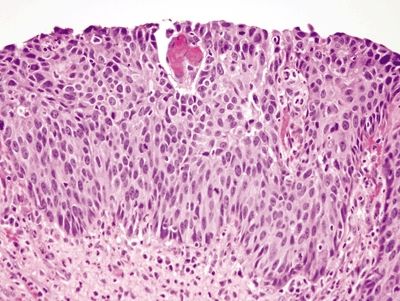
Figure 1.1.6 Squamous dysplasia. This is high-grade squamous dysplasia. It is difficult to see cell borders, and the nuclei are hyperchromatic. Note that cell borders are readily identifiable in the reparative squamous epithelium in Figure 1.1.2.
1.2 Reactive multinucleated squamous cells vs. Herpes virus cytopathic effect
| Reactive Multinucleated Squamous Cells | Herpes Simplex Esophagitis | |
|---|---|---|
| Age/Gender | Typically adults (mean age about 60); male predominance | Immunosuppressed persons of all ages, typically adults; no gender predilection |
| Location | Usually distal esophagus | Throughout esophagus |
| Symptoms | Since associated with esophageal mucosal injury, related to strictures, gastroesophageal reflux disease, dysphagia, odynophagia, heartburn, abdominal pain, gastrointestinal bleeding | Odynophagia, dysphagia |
| Signs | Endoscopically, ulcers, erosions, strictures, and mucosal plaques can all be seen | Vesicles (early) or ulcers (late). The endoscopist must biopsy squamous epithelium (rather than ulcer bed) to detect organisms |
| Etiology | Presumed to be reparative | Infection with herpes simplex virus |
| Histology | ||
| 1. Often seen at squamocolumnar junction with or without accompanying Barrett esophagus (with goblet cells) (Fig. 1.2.1) 2. May be accompanied by pseudoepitheliomatous hyperplasia (Fig. 1.2.2) 3. The nuclei in the multinucleated cells typically have prominent nucleoli but not true inclusions (Fig. 1.2.3) | 1. Ulcers and erosions are seen. The infected cells are seen at the epithelialized edges of the ulcers (Fig. 1.2.4) 2. Infected cells are either multinucleated or have a single nucleus (Fig. 1.2.5) 3. Both Cowdry A (with a clearing around the inclusion) and Cowdry B (smudged) inclusions can be seen within nuclei (Fig. 1.2.6) | |
| Special studies |
|
|
| Treatment | Treatment is of the underlying condition that resulted in the reactive changes | Oral antivirals such as acyclovir, famciclovir, and valacyclovir may be used. Intravenous acyclovir is used for individuals who cannot swallow due to odynophagia, persons with other systemic manifestations of herpes, or severely immunocompromised individuals |
| Prognosis | The prognosis is related to the underlying condition, typically reflux disease, but unrelated to the multinucleated cells | Good for the herpes itself—the root cause of the immunosuppression must be addressed to prevent reinfection |
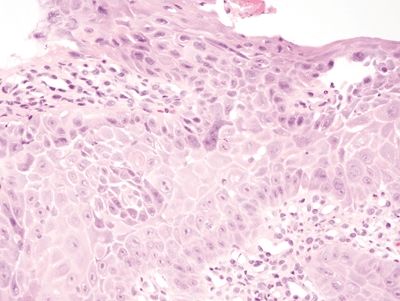
Figure 1.2.1 Reactive multinucleated squamous cells. The multinucleated cell in the center of the field has the same reparative changes as those in the cells with only one nucleus. It is at the basal layer.
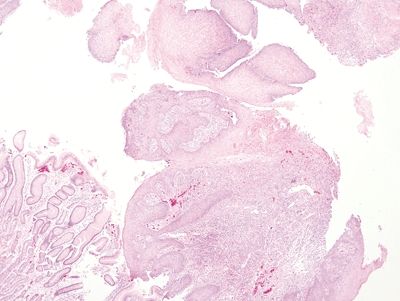
Figure 1.2.2 Reactive multinucleated squamous cells. At low magnification, note the lamina propria granulation tissue. A fragment of gastrocardiac mucosa is present. These samples were from the gastroesophageal junction of a patient with erosive reflux disease.
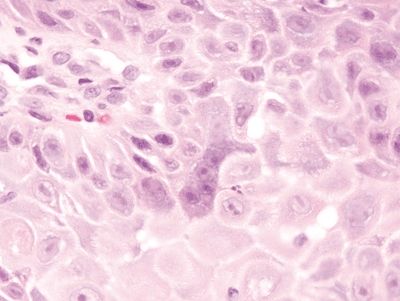
Figure 1.2.3 Reactive multinucleated squamous cells. Note the nucleoli in the reactive cells. Note also how prominent the intercellular bridges appear in this reactive squamous epithelium, a finding attributable to edema.
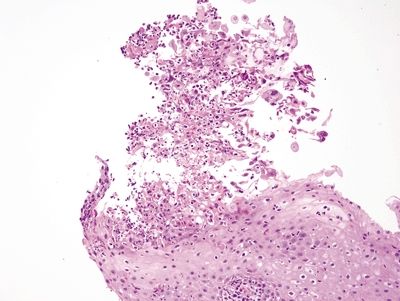
Figure 1.2.4 Herpes esophagitis. The infected cells are sloughed at the surface.
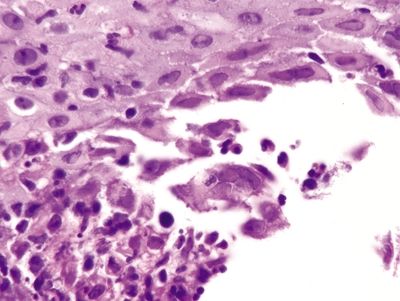
Figure 1.2.5 Herpes esophagitis. A multinucleated cell with smudged nuclei is in the center of the field. Similar binucleate cells are nearby. Note the sloughed debris at the lower left.
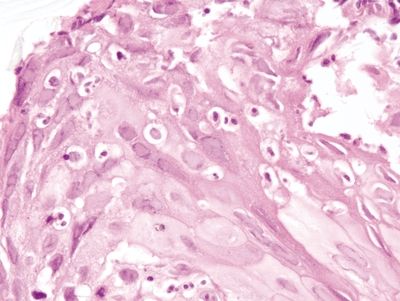
Figure 1.2.6 Herpes esophagitis. Both eosinophilic and basophilic smudged Cowdry B inclusions are present.
1.3 Reactive stromal changes vs. Cytomegalovirus esophagitis
| Reactive Stromal Changes in Ulcers and Polyps | Cytomegalovirus Esophagitis | |
|---|---|---|
| Age/Gender | Older individuals, often with comorbidities that might result in ischemia; no gender predilection | Typically adults; no gender predilection |
| Location | Anywhere in esophagus as associated with ulcers | No specific location in esophagus |
| Symptoms | Related to the ulcers—dysphagia, odynophagia | Odynophagia, dysphagia |
| Signs | Ulcer visible endoscopically | Endoscopists often see ulcers and must biopsy some of the ulcerated portion in order to maximize the likelihood of detection |
| Etiology | Whatever the etiology of the ulcer; reflux associated, medication associated, chemoradiation associated; often in debilitated persons | Cytomegalovirus, often found in immunocompromised persons regardless of the etiology of the immunocompromise. The patient may have evidence of systemic cytomegalovirus infection |
| Histology | ||
| 1. Markedly atypical fibroblasts that are found at the interface of necrotic ulcer exudate and viable granulation tissue (Fig. 1.3.1) 2. The fibroblasts have enlarged smudged nuclei but a low nuclear-to-cytoplasmic ratio; a pseudosarcomatous appearance (Fig. 1.3.2) 3. The fibroblasts intercalate among capillaries (Fig. 1.3.3) | 1. Erosions, granulation tissue typical (Fig. 1.3.4) 2. Often a background of monohistiocytic cells that can result in consideration of lymphoma (Fig. 1.3.5) 3. Both nuclear and cytoplasmic inclusions can be encountered. The infected cells are typically endothelial rather than epithelial cells (Fig. 1.3.6) 4. Immunolabeling can be used if there is uncertainty (Fig. 1.3.7) | |
| Special studies |
|
|
| Treatment | None for the fibroblasts themselves. Any treatment is directed at the underlying etiology of the ulcer (generally gastroesophageal reflux disease) | Foscarnet and ganciclovir appear to be similarly effective and safe |
| Prognosis | Excellent for the atypical stromal cells. The underlying condition responsible for the ulcers must be addressed | Cytomegalovirus esophagitis responds to antiviral treatment, but the underlying cause of immunosuppression needs to be addressed if feasible |
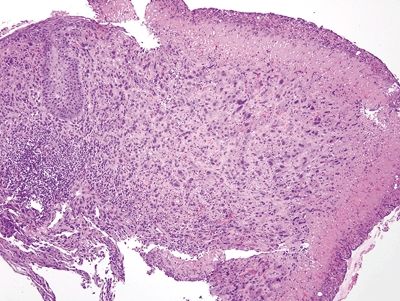
Figure 1.3.1 Pseudosarcomatous stromal cells in ulcers. Note that the atypical cells form a rind at the junction between the necroinflammatory cap at the right side of the fiend and the underlying healthy tissue.
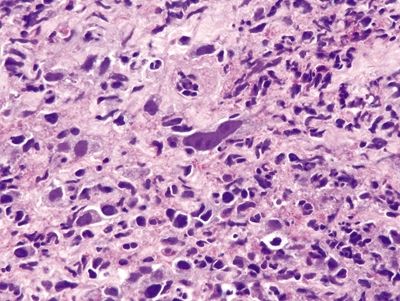
Figure 1.3.2 Pseudosarcomatous stromal cells in ulcers. The large cell in the center is a pseudosarcomatous fibroblastic cell. It has abundant amphophilic cytoplasm and nucleoli.
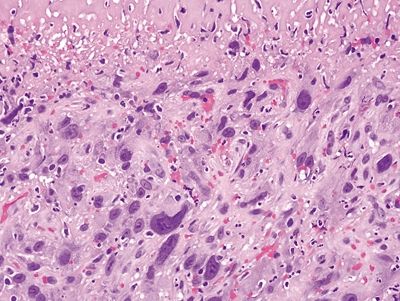
Figure 1.3.3 Pseudosarcomatous stromal cells in ulcers. At the interface between the necrotic surface and the viable underlying tissue, the atypical fibroblasts proliferate between capillaries.
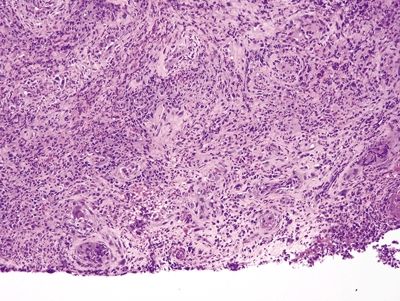
Figure 1.3.4 Cytomegalovirus esophagitis. This process has reactive fibroblasts but also shows viral cytopathic effect. At low magnification, a virocyte can be seen at the lower left.
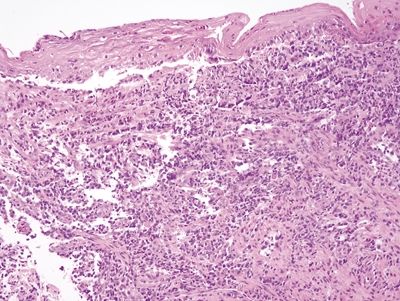
Figure 1.3.5 Cytomegalovirus esophagitis. This example shows a prominent lymphomonocytic infiltrate that raises the possibility of lymphoma. In such cases, it can be difficult to detect viral cytopathic effect.
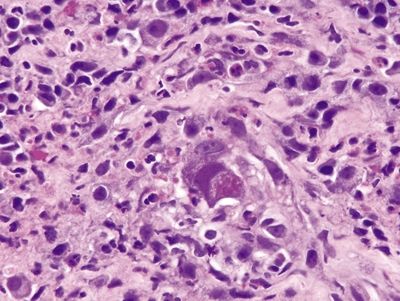
Figure 1.3.6 Cytomegalovirus esophagitis. Classic viral cytopathic effect. Both nuclear and cytoplasmic inclusions are present.
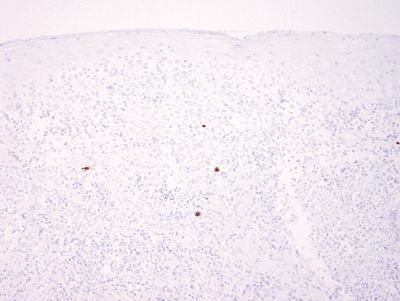
Figure 1.3.7 Cytomegalovirus esophagitis. This is an immunostain for cytomegalovirus on the area shown in Figure 1.3.5.
1.4 Goblet cells in Barrett esophagus vs. Multilayered epithelium
| Goblet Cells of Barrett Esophagus | Multilayered Epithelium in the Esophagus | |
|---|---|---|
| Age/Gender | Typically adults; strong male predominance | Adults. Median age in the initial immunolabeling series reporting this finding: 57 years. Male predominance |
| Location | Distal esophagus | Distal esophagus |
| Symptoms | None attributable to the goblet cells per se. The patient may have symptoms of gastroesophageal reflux such as heartburn or cough | No symptoms attributable to the multilayered epithelium per se. This finding does correlate with gastroesophageal reflux, and it may be a precursor to conventional Barrett mucosa. Patients can manifest symptoms of gastroesophageal reflux |
| Signs | Endoscopic “tongues” or “islands” of salmon-colored mucosa (columnar appearance) proximal to the gastric folds are seen in Barrett mucosa | None. Usually found on biopsies from the gastroesophageal junction |
| Etiology | Long-standing gastroesophageal reflux | Associated with reflux |
| Histology | ||
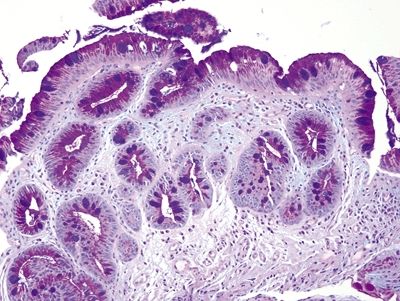
| 1. Typically incomplete intestinal metaplasia showing interspersed goblet cells and gastric foveolar-type cells. Goblet cells are characterized by an acid mucin vacuole that indents the nucleus in a cup shape. Nuclei are in more or less a monolayer and only minimally stratified (Figs. 1.4.1 and 1.4.2). Occasional examples show complete intestinal metaplasia with goblet cells interspersed between absorptive cells with a brush border (Figs. 1.4.3 and 1.4.4) 2. PAS/AB staining shows a dark blue to purple appearance of goblet cells with neutral mucin in gastric foveolar-type cells between the goblet cells in incomplete intestinal metaplasia (Fig. 1.4.5) or no mucin in absorptive cells of complete intestinal metaplasia (Fig. 1.4.6) | 1. This epithelium consists of four to eight layers of cells that appear to be squamous in the basal aspect and columnar in the superficial portion with an appearance reminiscent of that of immature squamous metaplasia of the uterine cervix (Figs. 1.4.7–1.4.10) 2. PAS/AB shows a purple in cells that appear similar to conventional goblet cells in fully columnar epithelium (Figs. 1.4.11 and 1.4.12) | |
| Special studies |
|
|
| Treatment | None currently generally suggested. Some observers have suggested radiofrequency ablation for nondysplastic Barrett esophagus, but this is not recommended for most patients | None |
| Prognosis | Most examples do not progress to adenocarcinoma, but Barrett esophagus is a precursor to esophageal adenocarcinoma | Multilayered epithelium is correlated with gastroesophageal reflux disease and may progress to conventional Barrett mucosa. It does not itself appear to be associated with dysplasia or adenocarcinoma in the absence of progression to conventional Barrett mucosa |
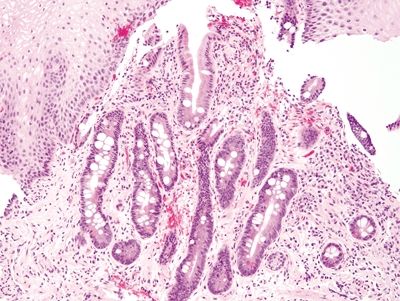
Figure 1.4.1 Barrett esophagus. This example shows a mixture of complete and incomplete intestinal metaplasia.
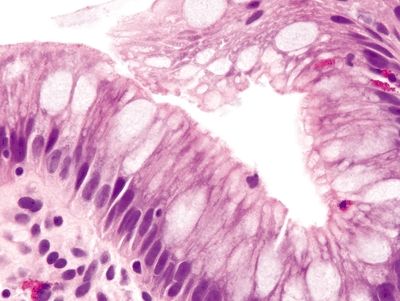
Figure 1.4.2 Barrett esophagus. This example shows incomplete intestinal metaplasia. There are cells producing neutral mucin between goblet cells.
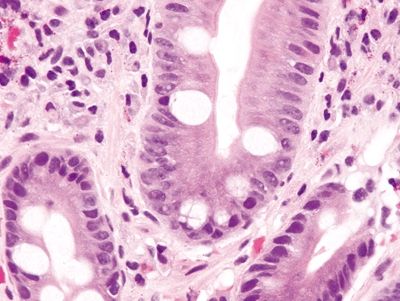
Figure 1.4.3 Barrett esophagus. This case shows complete intestinal metaplasia. There are cells that have a brush border between the goblet cells.
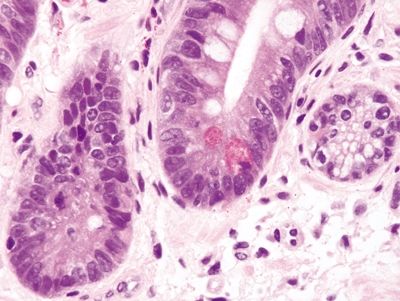
Figure 1.4.4 Barrett esophagus. This field shows complete intestinal metaplasia. Paneth cells are present.
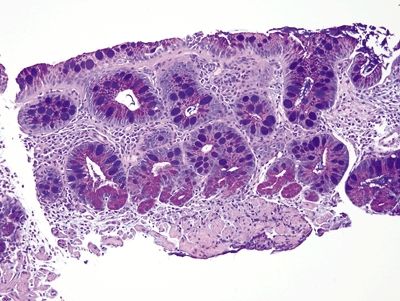
Figure 1.4.5 Barrett esophagus. This is a periodic acid Schiff–Alcian blue (PAS/AB) stain of incomplete intestinal metaplasia. The foveolar cells contain magenta neutral mucin, and the goblet cells are alcianophilic.
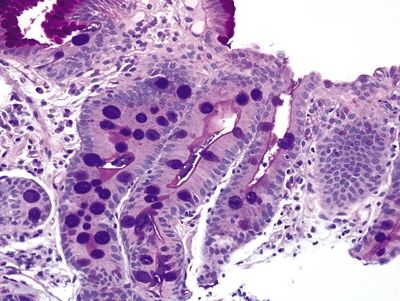
Figure 1.4.6 Barrett esophagus. This is a periodic acid Schiff–Alcian blue (PAS/AB) stain of complete intestinal metaplasia. There are no foveolar cells admixed with the goblet cells (although some are present on the surface of the mucosa), and the goblet cells are alcianophilic. There is a magenta strip on the surface of the cells between the goblet cells—the brush border.
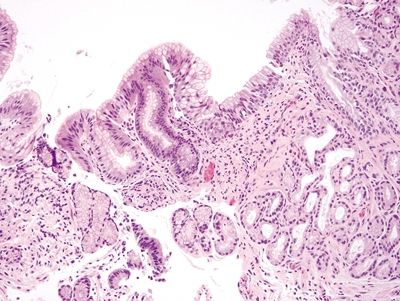
Figure 1.4.7 Multilayered epithelium. There is a stratified area of multilayered epithelium in a “U”-shaped configuration at the surface.
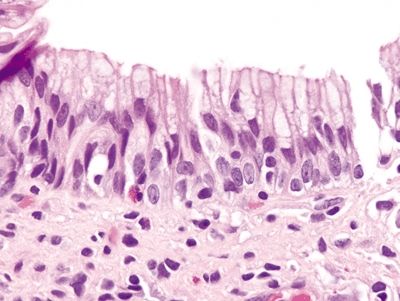
Figure 1.4.8 Multilayered epithelium. The deep portion resembles immature squamous metaplasia of the uterine cervix. The more superficial cells contain bubbly mucin reminiscent of the acid mucin seen in goblet cells of intestinal epithelium.
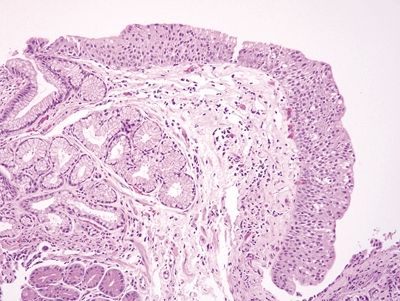
Figure 1.4.9 Multilayered epithelium. This example has more layers of stratification than that seen in Figure 1.4.7.
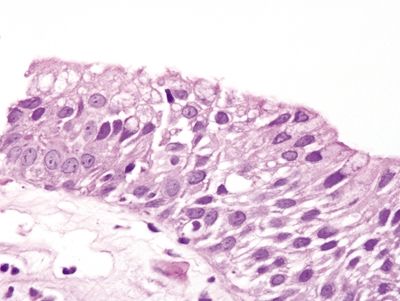
Figure 1.4.10 Multilayered epithelium.
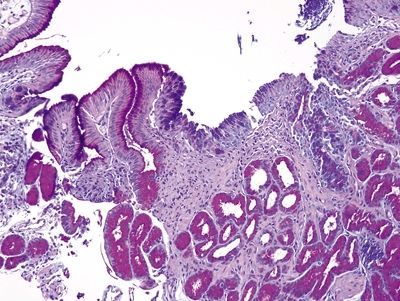
Figure 1.4.11 Multilayered epithelium. PAS/AB staining shows staining that is tinctorially similar to that in goblet cells at the surface of the multilayered area.
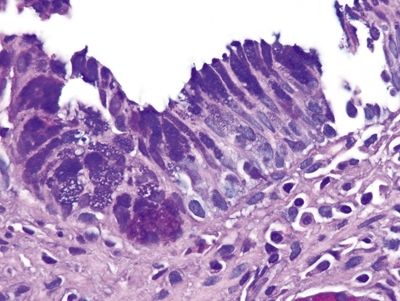
Figure 1.4.12 Multilayered epithelium. The globules of acid mucin (the mucin that is purplish on the PAS/AB stain) are not as crisply demarcated as are those in the goblet cells in Figures 1.4.5 and 1.4.6.
1.5 Goblet cells of Barrett mucosa vs. Esophageal submucosal glands
| Goblet Cells of Barrett Esophagus | Esophageal Submucosal Glands | |
|---|---|---|
| Age/Gender | Typically adults; strong male predominance | Normal anatomic structure found in all persons regardless of age/gender |
| Location | Distal esophagus | Throughout esophagus. These glands provide lubrication for the passage of swallowed food from the esophagus to the stomach |
| Symptoms | None attributable to the goblet cells per se. The patient may have symptoms of gastroesophageal reflux such as heartburn or cough | None |
| Signs | Endoscopic “tongues” or “islands” of salmon-colored mucosa (columnar appearance) proximal to the gastric folds are seen in Barrett mucosa | None—these are normal structures |
| Etiology | Long-standing gastroesophageal reflux | Unknown |
| Histology | ||
| 1. Goblet cells in Barrett esophagus are found in the surface epithelium and not in the submucosa. They are usually interspersed with gastric foveolar-type cells that contain sharply delineated mucin at the apex of the cell that does not indent the nucleus (Figs. 1.5.1–1.5.3) 2. PAS/AB staining shows a dark blue to purple appearance of goblet cells with neutral mucin in gastric foveolar-type cells between the goblet cells (Figs. 1.5.4–1.5.6) | 1. As per their designation as “submucosal glands,” esophageal submucosal glands are found in the submucosa rather than the mucosa, and they are therefore not seen on most esophageal mucosal biopsies. They are commonly found on endoscopic mucosal resection samples or submucosal dissection samples. They consist of glands arranged in lobules with plump cells containing abundant microvesicular mucin that appears pale on H&E staining (Figs. 1.5.7–1.5.9) 2. PAS/AB staining shows dark purple to blue glands with a monotonous color situated in the submucosa (Figs. 1.5.10–1.5.12) | |
| Special studies |
|
|
| Treatment | None currently generally suggested. Some observers have suggested radiofrequency ablation for nondysplastic Barrett esophagus, but this is not recommended for most patients | Not applicable—normal structures |
| Prognosis | Most examples do not progress to adenocarcinoma, but Barrett esophagus is a precursor to esophageal adenocarcinoma | Not applicable—normal structures |
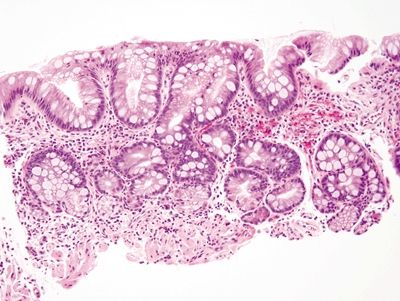
Figure 1.5.1 Barrett mucosa showing incomplete intestinal metaplasia. Goblet cells are interspersed with cells containing apical mucin.
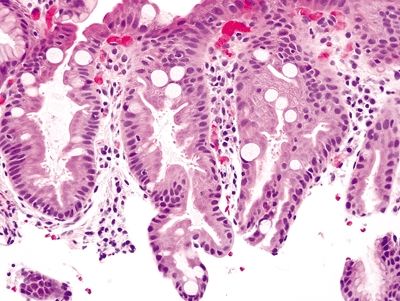
Figure 1.5.2 Barrett mucosa showing complete intestinal metaplasia. Goblet cells are interspersed with cells with a brush border.
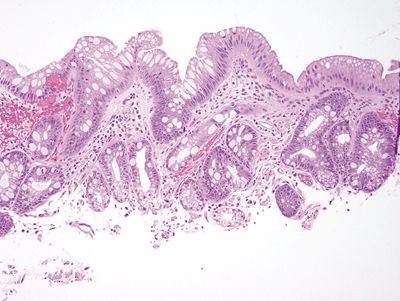
Figure 1.5.3 Barrett mucosa showing incomplete intestinal metaplasia.
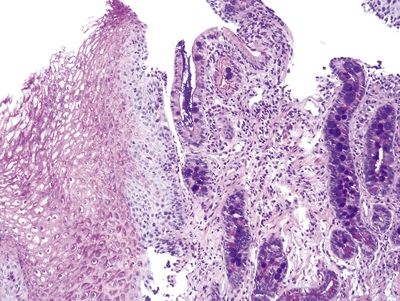
Figure 1.5.4 Barrett mucosa with both complete and incomplete intestinal metaplasia. Some areas show a brush border between goblet cells, whereas others show cells containing neutral mucin (PAS/AB).
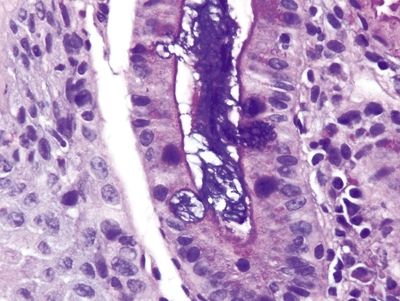
Figure 1.5.5 Barrett mucosa showing complete intestinal metaplasia. Note the brush border.
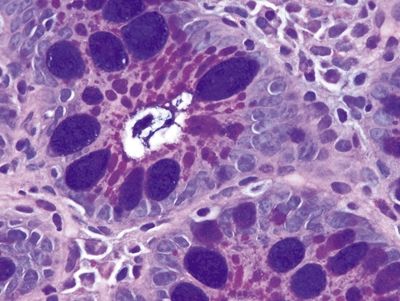
Figure 1.5.6 Barrett mucosa showing incomplete intestinal metaplasia.
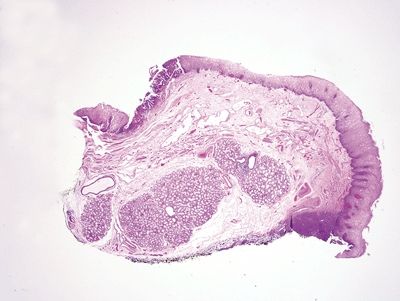
Figure 1.5.7 Esophageal submucosal glands, endoscopic mucosal resection specimen. This shows large submucosal glands in the deep portion of the biopsy. The upper left of the field shows an area of high-grade columnar epithelial dysplasia.
Figure 1.5.8 Esophageal submucosal glands. These glands are usually not encountered on biopsies of squamous mucosa, but occasionally they are present. They contain abundant bubbly mucin.
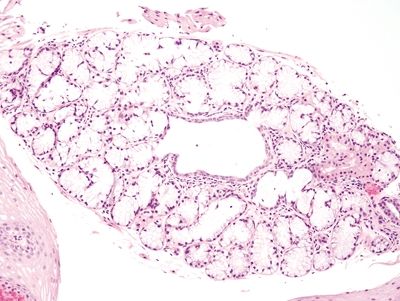
Figure 1.5.9 Esophageal submucosal glands.
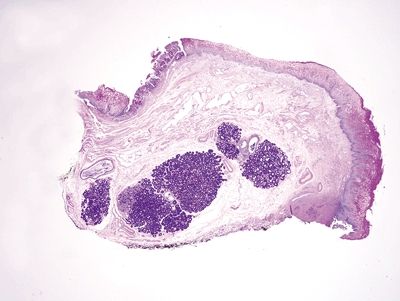
Figure 1.5.10 Esophageal submucosal glands, endoscopic mucosal resection specimen. Note the dark purple appearance of esophageal submucosal glands on PAS/AB.
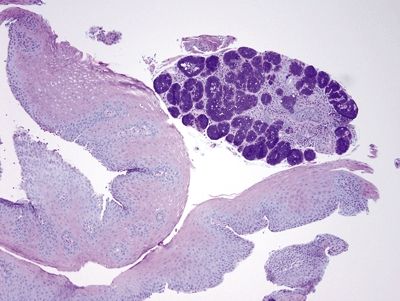
Figure 1.5.11 Esophageal submucosal gland, biopsy, PAS/AB.
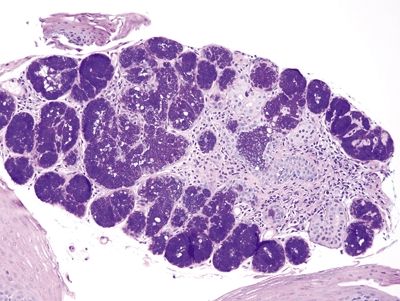
Figure 1.5.12 Esophageal submucosal gland, biopsy, PAS/AB.
1.6 Goblet cells of Barrett mucosa vs. Alcianophilic foveolar cells
| Goblet Cells of Barrett Esophagus | Alcianophilic Columnar Cells in the Esophagus | |
|---|---|---|
| Age/Gender | Typically adults; strong male predominance | Typically adults; strong male predominance |
| Location | Distal esophagus | Distal esophagus |
| Symptoms | None attributable to the goblet cells per se. The patient may have symptoms of gastroesophageal reflux such as heartburn or cough | None attributable to the columnar cells per se. The patient may have symptoms of gastroesophageal reflux such as heartburn or cough |
| Signs | Endoscopic “tongues” or “islands” of salmon-colored mucosa (columnar appearance) proximal to the gastric folds are seen in Barrett mucosa | Endoscopic salmon-colored mucosa (columnar appearance) proximal to the gastric folds can be seen as a corollary to any type of columnar mucosa |
| Etiology | Long-standing gastroesophageal reflux | Long-standing gastroesophageal reflux |
| Histology | ||
| 1. Goblet cells in Barrett esophagus are usually interspersed with gastric foveolar-type cells that contain sharply delineated mucin at the apex of the cell that does not indent the nucleus. Usually, goblet cells in Barrett esophagus are separated from one another by one to three cells that have the appearances of gastric foveolar in incomplete metaplasia or by one to three cells that have absorptive qualities in complete intestinal metaplasia (Figs. 1.6.1 and 1.6.2) 2. PAS/AB staining shows a dark blue to purple appearance of goblet cells with neutral mucin in gastric foveolar-type cells between the goblet cells or a brush border between the goblet cells in complete intestinal metaplasia (Figs. 1.6.3 and 1.6.4) | 1. In the United States and much of Europe, the presence of goblet cells is required to diagnose Barrett mucosa so Alcian blue (AB) staining is often performed with or without PAS. In contrast, only columnar epithelium is required for a diagnosis of Barrett esophagus in the United Kingdom and Japan. As such, in the United States, some examples of columnar epithelium lacking goblet cells display a bluish appearance on Alcian blue that appears similar to Barrett goblet cells. These alcianophilic cells often appear as several mucin-containing cells adjoining one another rather than separated by obvious foveolar or absorptive cells (Figs. 1.6.5 and 1.6.6) 2. PAS/AB staining shows tall blue cells that are usually adjacent to similar cells (Figs. 1.6.7 and 1.6.8) | |
| Special studies |
|
|
| Treatment | None currently generally suggested. Some observers have suggested radiofrequency ablation for nondysplastic Barrett esophagus, but this is not recommended for most patients | None currently generally suggested |
| Prognosis | Most examples do not progress to adenocarcinoma, but Barrett esophagus is a precursor to esophageal adenocarcinoma | Probably less likely to progress to adenocarcinoma than Barrett mucosa with classic goblet cells, at least in data from the United States |
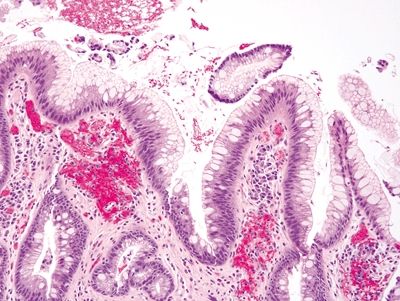
Figure 1.6.1 Barrett esophagus with incomplete intestinal metaplasia. Between the goblet cells, each of which has a crisply delineated mucin droplet that appears slightly bluish on H&E, there are foveolar cells containing apical mucin.
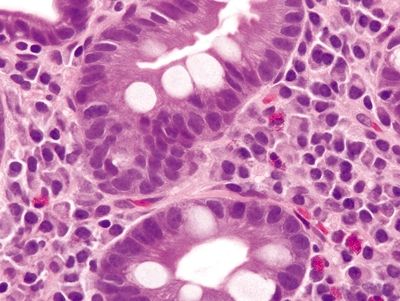
Figure 1.6.2 Barrett esophagus with complete intestinal metaplasia. Note the brush border of the absorptive-type cells between goblet cells.
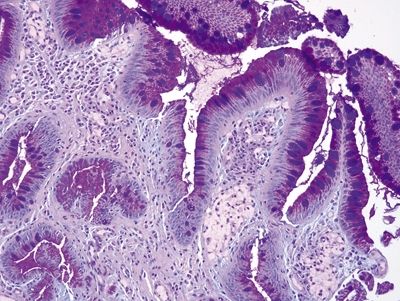
Figure 1.6.3 Barrett esophagus with incomplete intestinal metaplasia, PAS/AB stain. There are magenta-colored foveolar-type cells between the purple goblet cells.
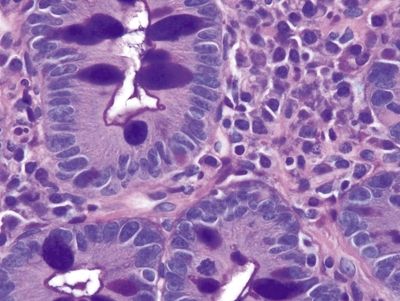
Figure 1.6.4 Barrett esophagus with complete intestinal metaplasia, PAS/AB stain. There is a sharp thin brush border that is dark blue between the goblet cells.
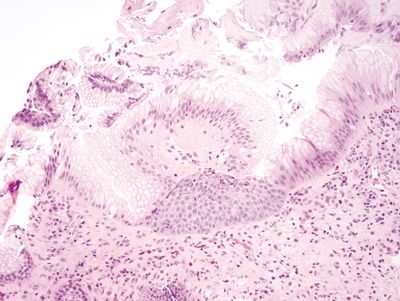
Figure 1.6.5 “Alcianophilic” foveolar cells in esophageal biopsies. The cells are reminiscent of goblet cells, but their mucin is the same color as that of foveolar cells.
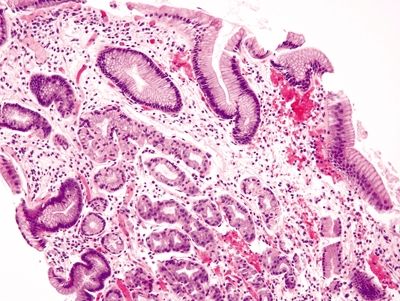
Figure 1.6.6 “Alcianophilic” foveolar cells in esophageal biopsies . This biopsy shows cardio-oxyntic mucosa lacking goblet cells.
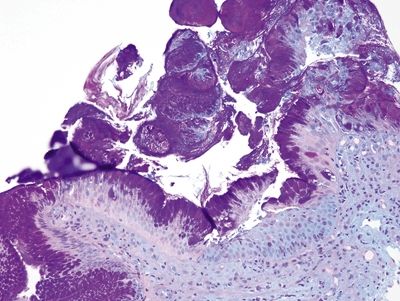
Figure 1.6.7 “Alcianophilic” foveolar cells in esophageal biopsies, PAS/AB stain. This is the same field as that shown in Figure 1.6.5. The purple cells do not correspond to goblet cells.
Figure 1.6.8 “Alcianophilic” foveolar cells in esophageal biopsies, PAS/AB stain. This cardio-oxyntic mucosa contains purple cells. These are not goblet cells and should not be reported as such.
1.7 Carryover of small bowel mucosa into esophageal sample vs. Barrett mucosa
| Carryover of Small Intestinal Mucosa into Esophageal Samples | Barrett Mucosa | |
|---|---|---|
| Age | Any age | Typically adults; strong male predominance |
| Location | Frequently, when upper gastrointestinal tract endoscopy is performed, endoscopists introduce the endoscope into the small bowel and retract the endoscope and take biopsy samples as they remove the endoscope. Thus, often the duodenum is biopsied first, followed my other biopsies in the upper gastrointestinal tract. When a biopsy is taken, the forceps are rinsed into a container containing formalin, then washed with saline, and then reinserted to take additional sample/s. Sometimes, if the esophagus is biopsied after the duodenum, a portion of duodenal tissue can remain adherent to the forceps and finally dislodge into the specimen jar intended for a subsequent esophagus sample. Also, occasionally, based on operator error, a sample from the duodenum can be submitted in a specimen container labeled “esophagus” | Distal esophagus |
| Symptoms | Unrelated to the sample | None attributable to the Barrett mucosa per se. The patient may have symptoms of gastroesophageal reflux such as heartburn or cough |
| Signs | A clue is that the pathologist may find goblet cells in the esophagus in the absence of a clinical impression of Barrett esophagus | Endoscopic “tongues” or “islands” of salmon-colored mucosa (columnar appearance) proximal to the gastric folds are seen in Barrett mucosa |
| Etiology | Operator error in labeling specimen site or adherence of a duodenal sample to forceps and deposit of this sample into a specimen container labeled “esophagus” | Long-standing gastroesophageal reflux |
| Histology | ||
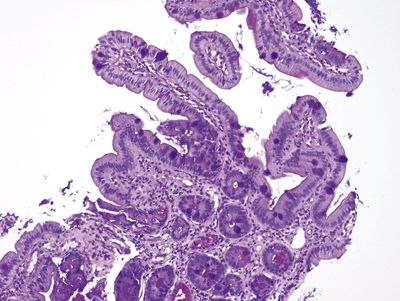
| 1. Among a sample showing histomorphology of gastroesophageal junction (gastrocardiac and squamous fragments), a separate fragment shows goblet cells, a perfect brush border, numerous lamina propria plasma cells, and numerous Paneth cells at the bases of the crypts. In some examples, the sample is superficial and may not demonstrate the Paneth cells (Figs. 1.7.1–1.7.3) 2. PAS/AB staining shows a perfect brush border (Fig. 1.7.4) | 1. Barrett esophagus contains goblet cells interspersed with gastric foveolar-type cells that contain sharply delineated mucin at the apex of the cell that does not indent the nucleus. This pattern has been termed “specialized columnar epithelium” or “Barrett esophagus” of the distinctive type since in years past in the United States, all columnar mucosa of the esophagus was included in Barrett esophagus. Usually, goblet cells in Barrett esophagus are separated from one another by one to three cells that have the appearances of gastric foveolar in incomplete metaplasia or by one to three cells that have absorptive qualities in complete intestinal metaplasia (Figs. 1.7.5 and 1.7.6). However, even in complete intestinal metaplasia, the glands are less orderly than those of true small bowel and there are fewer Paneth cells in complete intestinal metaplasia 2. PAS/AB staining shows a dark blue to purple appearance of goblet cells with neutral mucin in gastric foveolar-type cells between the goblet cells or a brush border between the goblet cells in complete intestinal metaplasia (Figs. 1.7.7 and 1.7.8) | |
| Special studies |
|
|
| Treatment | None | None currently generally suggested. Some observers have suggested radiofrequency ablation for nondysplastic Barrett esophagus, but this is not recommended for most patients |
| Prognosis | Reflective of any actual pathology in the sample | Most examples do not progress to adenocarcinoma, but Barrett esophagus is a precursor to esophageal adenocarcinoma |
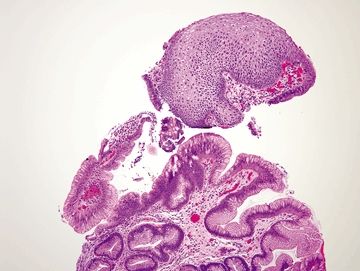
Figure 1.7.1 “Carryover” of a fragment of duodenal mucosa into a sample labeled “esophagus.” Note the tiny fragment in the center of the field. This patient had a duodenal biopsy followed by an esophagus biopsy that shows squamous and cardiac-type mucosa. A small fleck of duodenal mucosa became adherent to the forceps and finally dislodged into the esophagus sample cup. This is not Barrett mucosa.
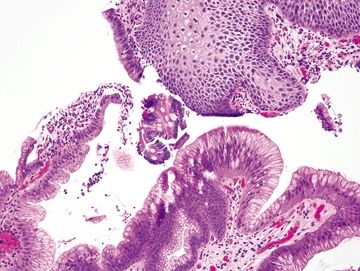
Figure 1.7.2 “Carryover” of a fragment of duodenal mucosa into a sample labeled “esophagus.” Higher magnification. Note the perfect brush border between the goblet cells.
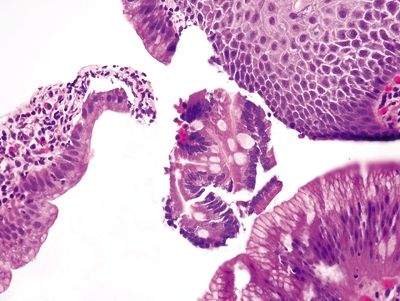
Figure 1.7.3 “Carryover” of a fragment of duodenal mucosa into a sample labeled “esophagus.” There are Paneth cells in addition to goblet cells in this fragment. It is unusual to have such perfect complete intestinal metaplasia in the esophagus. This sample was from a child with no endoscopic evidence of Barrett esophagus.
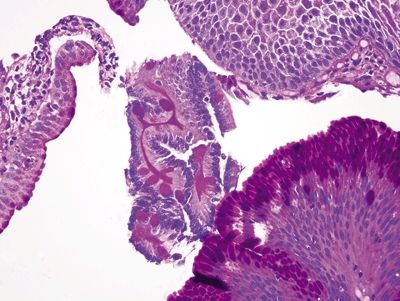
Figure 1.7.4 “Carryover” of a fragment of duodenal mucosa into a sample labeled “esophagus,” PAS/AB.
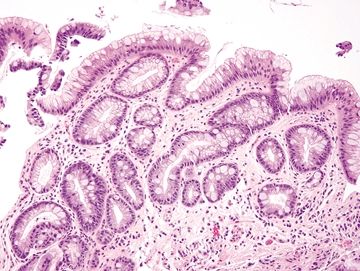
Figure 1.7.5 Barrett mucosa. There is incomplete intestinal metaplasia, and the fragment is sizable.
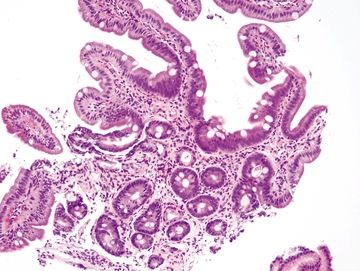
Figure 1.7.6 Barrett mucosa. Although there are villiform structures in this fragment that shows complete intestinal metaplasia (unusual for Barrett esophagus), there are no Paneth cells. The esophagus showed a tongue of columnar mucosa endoscopically.
1.8 Complete intestinal metaplasia vs. Incomplete intestinal metaplasia
| Complete Intestinal Metaplasia | Incomplete Intestinal Metaplasia | |
|---|---|---|
| Age/Gender | Adults; male predominance | Adults; male predominance |
| Location | Usually found in the stomach and less frequently found in the esophagus or other organs in the body that are not the focus of this book (bladder, pancreatobiliary system) | Usually found in the esophagus and less frequently found in the stomach or other organs in the body that are not the focus of this book (pancreatobiliary system) |
| Symptoms | Typically asymptomatic—any symptoms relate to the underlying source of injury that caused the intestinal metaplasia (usually Helicobacter pylori gastritis in the stomach and gastroesophageal reflux in the esophagus) | Typically asymptomatic—any symptoms relate to the underlying source of injury that caused the intestinal metaplasia (usually Helicobacter pylori gastritis in the stomach and gastroesophageal reflux in the esophagus) |
| Signs | None | None |
| Etiology | Many cycles of damage and repair of (usually) gastric mucosa | Many cycles of damage and repair of (usually) esophageal mucosa |
| Histology | ||
| 1. Goblet cells interspersed with absorptive columnar cells with a brush border in glands with minimal architectural distortion (Figs. 1.8.1 and 1.8.2). Paneth cells may be present 2. PAS/AB stain shows goblet cells separated by absorptive-type cells with an accentuated brush border (Figs. 1.8.3 and 1.8.4) | 1. Goblet cells interspersed with gastric foveolar-type cells in glands with moderate architectural distortion (Figs. 1.8.5 and 1.8.6) 2. PAS/AB stain shows goblet cells separated by neutral mucin-producing cells (Figs. 1.8.7 and 1.8.8) | |
| Special studies |
|
|
| Treatment | None | None |
| Prognosis | Lower but finite risk of entering the dysplasia–carcinoma sequence on epidemiologic grounds than incomplete intestinal metaplasia but not a prognostic marker in any individual case | Higher but finite risk of entering the dysplasia–carcinoma sequence on epidemiologic grounds than complete intestinal metaplasia but not a prognostic marker in any individual case |
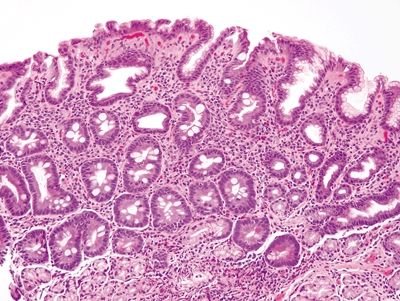
Figure 1.8.1 Complete intestinal metaplasia. This biopsy is actually from the gastric antrum rather than the esophagus since the antrum is a more likely location for complete intestinal metaplasia. There are goblet cells separated by absorptive cells, each with a brush border.
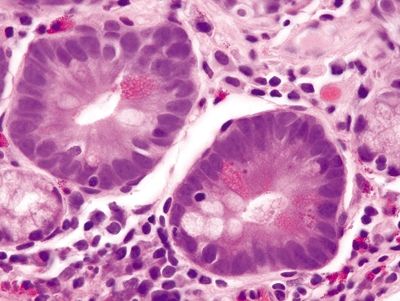
Figure 1.8.2 Complete intestinal metaplasia. This example shows goblet cells, a brush border, and even Paneth cells.
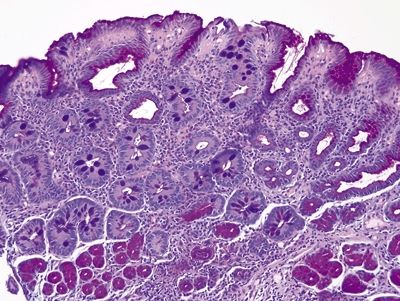
Figure 1.8.3 Complete intestinal metaplasia, PAS/AB stain. The gastric antral glands in the deep part of the biopsy contain pink neutral mucin as do the surface gastric foveolar cells. The complete intestinal metaplasia shows goblet cells between absorptive cells lacking cytoplasmic mucin.