Esophageal function tests are widely used, not only to obtain insight into esophageal physiology and pathophysiology in a research setting, but also to diagnose esophageal motor disorders in patients with symptoms such as dysphagia and chest pain. While esophageal function testing has long been considered almost synonymous with manometry, recently new techniques such as impedance measurement and high-resolution manometry have emerged. With impedance monitoring the transit of a bolus through the esophagus can be studied without the use of ionizing radiation. High-resolution manometry offers a highly detailed and comprehensive view of esophageal pressure patterns. Multichannel high resolution manometry with color plotting facilitates positioning of the catheter and interpretation of the tracings. In this article the development, clinical usefulness, and indications of these new tests are discussed.
The esophagus, a muscular tube with sphincters at each end, is the pathway between the pharynx and the stomach. The interplay between the muscular activity of the esophagus and its sphincters, coordinated by a network of nerves from the enteric and central nervous system, results in a fine-tuned system of peristalsis and sphincter relaxation that allows transport of contents toward the stomach with minimal stasis in the esophageal body. Furthermore, this system allows escape of gas from the stomach through the esophagus to the pharynx while reflux of liquid contents is swept back to the stomach by secondary peristalsis. Defects in this delicate system can lead to impaired bolus transport, muscular spasm, excess reflux of gastric contents, or impaired clearance of these refluxed contents. This can subsequently result in symptoms of dysphagia, chest pain, heartburn, and regurgitation.
Esophageal motility testing aims to clarify esophageal function and to reveal its disorders to explain a patient’s symptoms and to provide a rationale for therapy. However, the relationship between measured parameters and symptoms is often not very clear. On the one hand, esophageal motility disturbances do not always directly lead to symptoms and the clinical significance of observed motility abnormalities is often uncertain. On the other hand, many symptomatic subjects appear to have normal motility parameters. In part, this can be explained by the fact that esophageal motility is assessed using imperfect tools, such as esophageal manometry, which only provide indirect information on esophageal function. Theoretically, the ideal investigation provides all required information on esophageal motor function and allows accurate explanation of symptoms. Currently, patients with symptoms that suggest esophageal function defects are investigated with upper endoscopy, esophageal manometry, and videofluoroscopy using barium swallows. Although these techniques have shown to be clinically useful and are readily available, these studies provide no explanation for symptoms in a subset of patients.
Recently, two new techniques for assessment of esophageal function have been introduced: intraluminal multichannel impedance measurement and high-resolution manometry. We will review the principles of these new techniques and discuss whether they should become included in the diagnostic armamentarium of the twenty-first century gastroenterologist.
Measuring esophageal pressure
As indicated, the aim of esophageal motility tests is to assess the function of the esophagus and its sphincters to reveal abnormalities that can explain symptoms. The primary indication for esophageal manometry is nonobstructive dysphagia; that is, dysphagia for which no explanation can be found with other investigations. Indications for esophageal function testing include:
Nonobstructive dysphagia,
Unexplained chest pain,
Suspicion of generalized gastrointestinal motility disorder,
Before esophageal pH-(impedance) monitoring, and
Before anti-reflux surgery.
When esophageal manometry is performed to evaluate dysphagia, it is important to differentiate, based on the patient’s symptoms, between oropharyngeal or transfer dysphagia and esophageal dysphagia. With the former, the patient indicates that it is difficult to transfer food or drinks from the oral cavity to the esophagus or that aspiration occurs frequently. With the latter, the food does not go down after it has been swallowed. When the patient suffers from oropharyngeal dysphagia, the manometrist will have to pay special attention to the pharynx and the upper esophageal sphincter (UES). Pharyngeal hypoperistalsis, impaired UES relaxation, or insufficient coordination between pharyngeal contraction and UES can be found. In patients with esophageal dysphagia, stasis of the bolus in the esophageal body can be due to ineffective esophageal peristalsis or nontransmitted contractions. Furthermore, relaxation of the lower esophageal sphincter (LES) in response to a swallow can be inadequate. When absent peristalsis is found in combination with impaired LES relaxation, the diagnosis of achalasia can be made. Achalasia can easily be missed at upper endoscopy and, apart from establishing a diagnosis, manometry is important for the follow-up of achalasia and the response to therapy.
Esophageal manometry can also be used for the evaluation of patients with “noncardiac” chest pain; that is, episodic angina-like chest pain without an identifiable cardiac cause. Simultaneous contractions (spasms) can result in dysphagia but also in chest pain. Nutcracker esophagus is characterized by hypertensive contractions in the distal part of the esophageal body associated with chest pain. Although the yield of manometry for evaluation of chest pain is notoriously low, even when ambulatory 24-hour manometry is done, a positive diagnosis and subsequent explanation of the patient’s symptoms has a marked effect on his or her overall well-being.
Sometimes esophageal manometry is performed to evaluate whether a generalized gastrointestinal motility disorder (such as chronic idiopathic intestinal pseudo-obstruction) is present. Assessment of esophageal involvement in progressive systemic sclerosis and related conditions is another infrequent indication for esophageal manometry. Low-amplitude or even absent peristaltic contractions in the distal esophagus and low or absent LES pressure may be found.
There is no consensus on the role of esophageal manometry in the preoperative workup before antireflux surgery. The predictive value of IEM for postoperative dysphagia is poor, but it seems wise to hesitate on performing a fundoplication in a patient with severe peristaltic dysfunction.
As mentioned above, esophageal motility abnormalities can predispose to gastroesophageal reflux disease (GERD). Impaired peristalsis is related to poor clearance of esophageal contents and leads to prolonged presence of noxious acid material after a reflux episode. Furthermore, an impaired antireflux barrier with an absent LES pressure is likely to lead to a high number of reflux episodes. However, these abnormalities lack sensitivity and specificity and, therefore, esophageal manometry is not indicated in the diagnostic workup of patients with reflux symptoms. In our laboratory, the most common reason for performing esophageal manometry in patients presenting with reflux symptoms is the fact that manometry is the most accurate tool to identify the upper border of the LES before 24-hour reflux monitoring.
Development of High-Resolution Manometry
From the first esophageal pressure measurements until now, there has been a stepwise improvement of this technique. This was driven by a clinical demand for better equipment and by emerging technical possibilities. At first, a single-lumen catheter with one side hole was used for esophageal manometry and, using a pull-through maneuver, the entire esophagus could be covered. Soon, more perfused side holes were used to measure pressures at different positions in the esophagus simultaneously. With the pull-through technique, the LES and UES can be identified and resting pressures can be measured. Furthermore, peristalsis can be observed and the amplitude, duration, and velocity of the propulsive contractions can be studied. However, measurement of sphincter relaxation is difficult and unreliable with a single point sensor at the esophagogastric junction: longitudinal muscle contraction with swallowing results in upward movement of the LES and UES, simulating sphincter relaxation.
The introduction of the sleeve sensor by Dent made reliable LES relaxation measurements possible. The sleeve sensor is a perfused membrane, usually 6 cm long, positioned along the distal end of the catheter which records the highest pressure exerted along the membrane. Therefore, axial movement of the LES along the sleeve membrane will not influence pressure registration. The development of the sleeve sensor is a perfect example of how a technical improvement responded to a clinical demand. The current gold standard for esophageal manometry is a catheter with a sleeve sensor positioned at the esophagogastric junction and four to eight point sensors in the esophageal body.
In the 1990s, micromanometry, or high-resolution manometry, was introduced. Technical advances made it possible to manufacture small-caliber silicon rubber catheters with a large number of perfused channels (often > 20) and hydraulic flow restrictors with a very low perfusion rate. Most frequently, catheters with side holes placed at 1-cm intervals were used. Micromanometry made it possible to leave the catheter in position while studying esophageal peristalsis and LES function in great detail. Currently, both water-perfused and solid-state catheters (with up to 36 sensors at 1-cm intervals) can be used for high-resolution manometry.
Clouse and colleagues developed the concept of representing high-resolution pressure data in pseudo–three-dimensional and isobaric contour plots. In the latter, time and position in the esophagus are placed on the x- and y-axis respectively, and areas of equal pressure are represented by lines, as in a geographic altitude map. Because the number of channels was too low to span the entire esophagus, Clouse and colleagues used a pull-through technique to obtain a complete picture of esophageal pressure patterns.
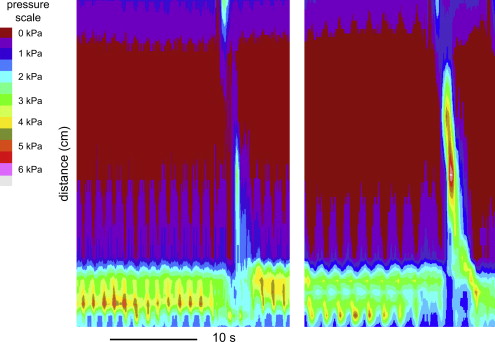
High-resolution manometry as it is performed today is made possible by combining modern micromanometric techniques and the topographic plotting method ( Fig. 1 ). In most plotting algorithms, in particular color plotting, interpolation of data is used to fill in the 1-cm gaps between the pressure sensors. This increases the visual attractiveness of the plot without adding new information. One of the advantages of modern high-resolution manometry is that pressures in the entire esophagus and its sphincters can be measured without having to perform multiple pull-throughs; therefore, the catheter can stay in place. Furthermore, the high spatial resolution at the esophagogastric junction assures that LES relaxation is measured adequately. In the following paragraphs, the clinical value of high-resolution manometry and topographic plotting is discussed.
High-Resolution Manometry
The first observations suggesting that conventional manometry is limited by a low spatial resolution were made by Clouse and colleagues. In their initial study they observed that esophageal peristalsis could be manometrically divided into three segments with two pressure troughs in between. The first trough, at the level of the aortic arch, is thought to represent the transition from striated to smooth muscle. The second trough, located two-thirds of the distance between the UES and the LES of the esophageal body, divided the smooth muscle part into two separate peristaltic waves. The observation of three rather distinct pressure zones in the esophageal body seemed to be a consistent and reproducible finding and it was shown that these zones showed a distinct response to cisapride. These studies were followed by a study of healthy volunteers and patients with nutcracker esophagus. In that study, the presence of two pressure troughs was confirmed in healthy subjects. However, in the nutcracker patients the second trough was not observed, probably owing to a marked increase in pressure in the distal smooth muscle segment. In later studies, the importance of the pressure trough between the striated and smooth muscle was stressed, and two distal pressure zones produced by smooth muscle contraction were regarded as a single functional segment. Although it has not been shown in a large group of patients yet, it has been observed that bolus escape during swallowing occurs frequently at the level of the pressure trough. It is hypothesized that the length of the pressure trough and coordination between striated and smooth muscle contractions plays a role in bolus transit abnormalities and the occurrence of dysphagia. It was indeed observed that high-resolution manometry was more accurate in predicting success of bolus transport than sleeve sensor manometry. In the same publication, several cases were presented in which high-resolution manometry revealed the cause of their symptoms while conventional manometry did not. These included a patient with an arteria lusoria and a patient with achalasia in which the LES relaxation was erroneously judged as normal using conventional manometry. In a subsequent study, it was shown that a 7-day course of the prokinetic tegaserod promoted midesophageal contractility and shortened the pressure transition zone. However, this had no effect on liquid bolus transport and only a trend toward significant improvement of solid bolus transport was observed.
A clinical study compared conventional four-sensor pull-through manometry with high-resolution manometry in 212 patients referred for esophageal manometry for various indications. Similar diagnostic criteria were used for conventional manometry and high-resolution manometry, and the length of the pressure trough and the presence of segmental spasm were not taken into account. In the majority of cases, (88.2%) there was diagnostic agreement between the two methods. However, the limited method failed to detect 6 of 36 achalasia patients that were detected by high-resolution manometry. Furthermore, the limited method could not select 12 patients with incomplete LES relaxation that were identified with high-resolution manometry. In 8 of these 12 patients, clinical data during follow-up supported the findings of inappropriate LES relaxation. Taken together, it was concluded that high-resolution manometry is a more sensitive technique compared with limited conventional manometry. The results of this study are important. It is the first effort to compare high-resolution manometry and conventional manometry and its findings suggest that the former is superior to the latter technique. The main difference between the two techniques is that with high-resolution manometry impaired LES relaxation is better recognized than pull-trough manometry. However, it should be borne in mind that impaired LES relaxation cannot be detected accurately with a pull-through manometry. It is, therefore, uncertain whether high-resolution would also have been better than sleeve sensor manometry.
In addition to allowing assessment of pressure troughs and LES relaxation, high-resolution manometry also makes it easier to assess simultaneous contractions and esophageal pressurization events. Studies on double-peaked contraction waves, which can be observed in patients with dysphagia and chest pain, reveal that these contraction waves often consist of an initial peristaltic contraction of the proximal smooth muscle segment followed by a short non-propulsive contraction in the distal smooth muscle segment. The clinical significance of these double-peaked contraction waves may be uncertain when observed during conventional manometry, as well as with high-resolution manometry. Only high-resolution manometry offers better understanding of what is actually happening.
Another abnormality with uncertain clinical significance that is relatively often observed with high-resolution manometry in patients with chest pain and dysphagia is the presence of segmental esophageal contractions (spasms). Future studies will have to clarify whether these findings bear relevance to individual patients or whether these are irrelevant motor abnormalities.
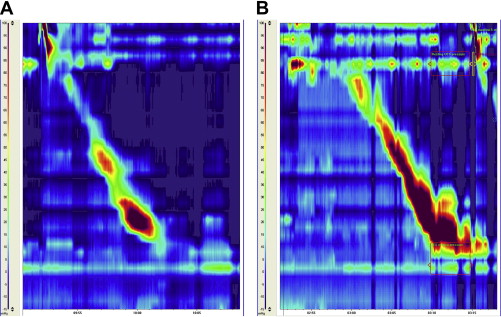
One of the most frequent indications for performing esophageal manometry is localization of the upper border of the LES, before positioning a catheter for ambulatory reflux monitoring. With conventional manometry, this is performed with a pull-through of the manometry catheter, until the LES can be recognized as a localized high-pressure zone at the esophagogastric junction. The pH catheter is subsequently positioned 5 cm above the upper border of the LES. While the LES can be accurately localized in most cases with this technique, it is less reliable in cases of a large hiatal hernia. In a normal anatomic situation, the LES and diaphragm together form the antireflux barrier, and the pressure zones of these two components overlap. However, in cases of a hiatal hernia, the LES is localized more proximally and the hiatal sac, a zone of lower pressure between the high-pressure zones of the LES and the diaphragm, is found. With conventional manometry, the high-pressure zone induced by the diaphragm can be mistaken for the LES, resulting in too distal placement of the pH catheter. This is even more likely when LES pressure is low, a situation encountered frequently in cases of a large hiatal hernia. Positioning the pH catheter below the LES will result in an overestimation of the acid exposure time. With high-resolution manometry, a pull-through is not required for localization of LES position because it can be immediately seen on the tracing. Even in case of a large hiatal hernia and an almost absent LES pressure, it is clear where the LES is positioned since this is also the level where peristaltic contraction waves end ( Fig. 2 ). This is also the result of the study by Clouse and colleagues on this subject. High-resolution manometry is definitely better for the identification of LES localization.
As mentioned, manometry can be performed on patients with oropharyngeal dysphagia. These patients may have pharyngeal hypoperistalsis, impaired coordination between pharyngeal contraction, and UES relaxation or incomplete UES relaxation. The UES and pharyngeal pump consist of striated skeletal muscle and muscle actions are rapid. Therefore, a fast frequency response system is required to measure their motor activities. Solid-state systems meet that requirement; perfusion systems do not. However, the axial movement of the sphincter during relaxation dictates that either a sleeve sensor or an array of closely spaced point sensors be used to adequately monitor pressure during this process. It is obvious that with conventional manometry these requirements cannot be met. High-resolution manometry seems ideally suited to study UES characteristics as it offers both a short response time and closely spaced pressure sensors, preempting movement artifacts. Recent publications have provided new insights into the physiology of UES relaxation. Using simultaneous high-resolution manometry and videofluoroscopy, the relationship between pharyngo-esophageal segment muscle activity and bolus transport can be studied in detail. In patients with a restricted UES opening, the pressure gradient measured in the bolus predicted the existence and location of abnormal constriction. As UES characteristics are always difficult to study with conventional manometry, it is likely that more and possibly clinically useful insights will be obtained with high-resolution manometry.
Development of Specific Analysis Algorithms for High-Resolution Manometry
Until recently, the analysis of most high-resolution manometry studies was performed in a manner similar to conventional manometry. For example, the highest pressure over the LES as measured with high-resolution manometry could be expressed by analysis software, just as it would have been measured with a conventional sleeve sensor measurement. This was performed by using an e-sleeve, which is a software-simulated sleeve sensor that measures the highest pressure recorded over a 6-cm long segment positioned at the esophagogastric junction, the raw data being read from an array of six point sensors. Because a similar analysis was used, it is not a complete surprise that the observed differences in diagnostic yield are not very large. However, the way in which data obtained by high-resolution manometry is presented allows for alternative means of analysis. Measurements of the transsphincteric pressure gradient to predict sphincter opening was the first attempt to develop a new way of analysis. This was followed by various reports describing efforts to develop new, more appropriate ways to perform the analysis of these tracings.
The Chicago group made particular efforts to introduce new parameters for dedicated analysis of high-resolution manometry data. They defined flow-permissive pressure as the intrabolus pressure that is required to overcome the pressure at the LES. The integrated relaxation resistance (IRR) was developed to express the period that the pressure at the LES was below a certain pressure. It can be calculated by creating a graph with the flow-permissive pressure on the horizontal axis and the duration of this pressure on the vertical axis. The relaxation interval is then defined as the time required to attain 95% of the plateau value. The IRR is calculated as the medium flow-permissive pressure divided by the relaxation interval. Bolus passage is expected to be impaired if the IRR is outside the 95th percentile of healthy subjects. Calculation of this IRR seems very complicated. Whereas it is uncertain whether it is an improvement over simple parameters such as LES nadir pressure or percent of relaxation, we still prefer these latter parameters as they are easier to comprehend. However, it is through endeavors such as these that the optimal technique for analysis of motility disorders will be established.
Other parameters that have been introduced are the distal contractile integral (DCI) and the pressurization front velocity (PFV). The PFV is calculated from the 30-mm Hg isobaric contour plots by marking the distal temporal margin of the transition zone and the superior margin of the esophagogastric junction and then calculating the slope between the two. It is a parameter similar to contraction velocity, which is often used in conventional manometry. The DCI quantifies the length, vigor, and persistence of the distal contractile wave. It is calculated by multiplying the distance between the transition zone and the proximal border of the LES, the time duration of the contraction and the integrated (summated) pressure amplitude, and is expressed as mm Hg·s·cm. A DCI higher than 5000 mm Hg·s·cm, which is the 95th percentile of 75 asymptomatic volunteers, is considered abnormal. The DCI and PFV can be calculated using dedicated software. As with the IRR, the DCI and PFV may be intuitively logical parameters. It has yet to be proven that these more complex parameters offer advantages above the simpler parameters that have been used in the past.
High-resolution manometry allows calculation of the trans-sphincteric pressure gradient. In the first study on this subject, the transsphincteric pressure gradient was calculated as the difference in pressure 2 cm above and 2 cm below the point where the LES was situated before relaxation. In rest, the pressure in the esophagus equals the intrathoracic pressure. This pressure is lower than the pressure in the stomach, which equals the pressure in the abdominal cavity. In case of complete LES relaxation, the transsphincteric pressure gradient becomes equal to zero as no barrier between the esophageal body and gastric cavity is left. This implies that impaired relaxation, such as occurs in patents with achalasia, can be detected with high-resolution manometry in a different way than by measuring LES pressure. The transsphincteric pressure gradient is also relevant for the occurrence of gastroesophageal reflux. It has been shown that the transsphincteric pressure gradient was greater during transient lower esophageal sphincter relaxations (TLESRs) with gastroesophageal reflux compared with TLESRs without evidence of reflux. However, this could not be confirmed by others.
High-resolution manometry makes it possible to study intrabolus pressure, an important indicator of the forces resisting peristaltic transport and the occurrence of ineffective bolus transport. A study in which swallows were observed in 12 patients with GERD, before and after fundoplication. It showed that after surgery restricted hiatal opening and impaired esophagogastric junction relaxation required a higher bolus pressure for adequate transit. This study illustrates the usefulness of this parameter and suggests potential clinical usefulness.
As high-resolution manometry displays pressures at small intervals in the esophagus, the presence of a hiatal hernia can easily be identified with this technique ( Fig. 3 ). Since the presence of a hiatal hernia is an important risk factor for GERD, assessment of this parameter may also have clinical value. During prolonged measurements of esophageal pressures in patients with a small hiatal hernia it was observed that the distance between LES and diaphragm was not stable, but varied over time. Sliding of the hernia could be monitored in real-time and it was observed that the hernia was intermittently reduced for a significant period of time. During periods in which the hernia was present there was an almost twofold higher prevalence of gastroesophageal reflux. After surgical correction of the hernia during a fundoplication, the manometric signs of hernia were no longer observed.