Esophageal Cancer: Clinical Management
J. Rüdiger Siewert
Michael Molls
Frank Zimmermann
Florian Lordick
In contrast to gastric cancer incidence, which is decreasing worldwide, the incidence and prevalence of esophageal carcinoma are rising at an alarming rate in the Western world. This rise is due primarily to an increase in the rate of adenocarcinoma of the distal esophagus (1). At many institutions in the Western world, adenocarcinomas of the esophagus now outnumber squamous cell esophageal cancers. Because of marked differences in the pathogenesis, tumor location, tumor biology, and characteristics of the affected patients (Table 18.1), squamous cell carcinoma and adenocarcinoma of the esophagus should be treated as separate entities to a certain extent (2). This differentiation is frequently not made when treatment results for esophageal cancer are reported.
Despite marked advances in the surgical treatment of squamous cell carcinoma and adenocarcinoma of the esophagus, the overall prognosis for affected patients has not improved much over the past decades. This is because these tumors continue to be diagnosed at an advanced stage in the majority of the affected patients. An improvement of early diagnosis in the Western Hemisphere is only visible in Barrett Ca. Furthermore, systemic and local recurrences are still common even after a complete tumor resection, extensive lymphadenectomy, or multidisciplinary approaches (2). In the future, a valuable improvement in the overall survival of patients with esophageal cancer most likely can be achieved by the development of tailored therapeutic strategies based on the individual histologic tumor type, tumor location, tumor stage at time of presentation, response to induction radiochemotherapy (RCT), and consideration of other established prognostic factors (2). A clear classification of the underlying tumor entity, a profound knowledge of the prognostic factors applicable, and a thorough preoperative staging are therefore essential for the selection of the optimal therapeutic modality in a given situation.
Topographic Classification
The classification of squamous cell esophageal cancer according to its location in the proximal, middle, and distal third of the esophagus should be abandoned. Rather, these tumors should be classified into tumors of the cervical esophagus (“cervical esophageal cancer”); tumors arising above the level of the tracheal bifurcation (“suprabifurcal esophageal cancer”), which means with contact to the tracheal-bronchial tree; and tumors arising below the level of the tracheal bifurcation (“infrabifurcal esophageal cancer”) (3), which means without contact to the tracheal-bronchial tree. The selection of treatment strategy is guided by this topographic classification. Whereas tumors located below the level of the tracheal bifurcation can frequently be resected with adequate margins, an extensive resection of transmural suprabifurcal or cervical tumors is usually prohibited by the proximity to the tracheoesophageal tree. The pattern of lymphatic spread also depends on the location of the primary tumor. The direction of lymphatic flow is primarily directed to the upper mediastinum and cervical region in patients with suprabifurcal tumors, and to the lower posterior mediastinum and celiac axis in patients with infrabifurcal tumors. Tumors located at the level of the tracheal bifurcation tend to metastasize in both directions (3,4).
An even better classification is based on a direct assessment of the relationship between the primary tumor and the tracheobronchial tree, that is, a differentiation of tumors with and without contact to the trachea or mainstem bronchi. These two classification systems are not identical because a tumor arising below the level of the tracheal bifurcation may still have contact with the tracheobronchial tree. In this situation, the area of contact is usually in the region of the left mainstem bronchus.
Adenocarcinoma of the esophagus, which is usually located in the distal esophagus, should be differentiated from other tumor entities arising in the vicinity of the esophagogastric junction. Due to the borderline location and the ambiguous use of the term cardia carcinoma, many discrepancies exist in the current literature regarding the classification of these tumors. Although some classify all such tumors as esophageal carcinomas, others classify them as gastric carcinomas or regard them as an entity separate from esophageal and gastric cancer. To clarify these issues, adenocarcinomas of the esophagogastric junction (AEG) are defined here as tumors that have their center within 5 cm proximal and distal of the anatomical cardia and have differentiated three distinct tumor entities within this area (5,6):
AEG type I—adenocarcinoma of the distal esophagus that usually arises from an area with specialized intestinal metaplasia of the esophagus (i.e., Barrett esophagus) and may infiltrate the esophagogastric junction from above
AEG type II—true carcinoma of the cardia arising from the cardiac epithelium or short segments with intestinal metaplasia at the esophagogastric junction
AEG type III—subcardial gastric carcinoma that infiltrates the esophagogastric junction and distal esophagus from below
The assignment to each type is purely morphologic and is based on the anatomical location of the tumor center or, in patients with advanced tumor, the location of the tumor mass.
This differentiation of esophagogastric junction tumors is supported by several observations. Patients with AEG type I tumors are more likely to have a hiatal hernia and a long history of gastroesophageal reflux disease than are patients with AEG type II or type III tumors. Specialized intestinal epithelial
metaplasia in the distal esophagus (Barrett esophagus) with subsequent development of progressively severe dysplastic changes has been clearly identified as the main precursor lesion for adenocarcinoma in the distal esophagus (AEG type I) (7,8). Although an association with short segments of intestinal metaplasia at or below the gastric cardia has also been reported for AEG type II and III tumors, this is rather uncommon (6). In addition, there appears to be a strong association between Helicobacter pylori infection and intestinal metaplasia at or below the gastric cardia; this is not the case for specialized intestinal metaplasia in the distal esophagus, which is clearly reflux related (8). Furthermore, the prevalence of undifferentiated tumors and tumors with a “nonintestinal” growth pattern is rather low in AEG type I tumors and increases significantly from AEG type II to AEG type III tumors (6). Accordingly, the expression of cytokeratins and cell adhesion molecules, as well as the prevalence and pattern ofgenetic abnormalities detected by comparative genomic hybridization, show marked differences among the three AEG tumor types (5). Finally, lymphographic studies indicate that the main lymphatic pathways originating from the lower esophagus advance both upward into the mediastinum and downward along the celiac axis, whereas those from the gastric cardia and subcardial region preferentially make their way to the celiac axis, the splenic hilus, and the para-aortic lymph nodes (9). This is reflected in the different patterns of lymphatic spread of the three tumor entities at the esophagogastric junction (6). The most important aspect (with therapeutical consequences) is the differentiation of Barrett Ca from gastric Ca. Some imaging is helpful for this differentiation. Based on these observations, which support a possible heterogeneity in the pathogenesis and biological behavior of the different tumor types, all experts at a consensus conference and the International Society for Diseases of the Esophagus agreed that this classification should form the basis for defining, assessing, and reporting treatment of adenocarcinoma arising in the vicinity of the esophagogastric junction (6) and differentiating adenocarcinoma of the distal esophagus from the other tumor entities. This classification is now accepted worldwide, and an increasing number of publications on this topic are based on it.
metaplasia in the distal esophagus (Barrett esophagus) with subsequent development of progressively severe dysplastic changes has been clearly identified as the main precursor lesion for adenocarcinoma in the distal esophagus (AEG type I) (7,8). Although an association with short segments of intestinal metaplasia at or below the gastric cardia has also been reported for AEG type II and III tumors, this is rather uncommon (6). In addition, there appears to be a strong association between Helicobacter pylori infection and intestinal metaplasia at or below the gastric cardia; this is not the case for specialized intestinal metaplasia in the distal esophagus, which is clearly reflux related (8). Furthermore, the prevalence of undifferentiated tumors and tumors with a “nonintestinal” growth pattern is rather low in AEG type I tumors and increases significantly from AEG type II to AEG type III tumors (6). Accordingly, the expression of cytokeratins and cell adhesion molecules, as well as the prevalence and pattern ofgenetic abnormalities detected by comparative genomic hybridization, show marked differences among the three AEG tumor types (5). Finally, lymphographic studies indicate that the main lymphatic pathways originating from the lower esophagus advance both upward into the mediastinum and downward along the celiac axis, whereas those from the gastric cardia and subcardial region preferentially make their way to the celiac axis, the splenic hilus, and the para-aortic lymph nodes (9). This is reflected in the different patterns of lymphatic spread of the three tumor entities at the esophagogastric junction (6). The most important aspect (with therapeutical consequences) is the differentiation of Barrett Ca from gastric Ca. Some imaging is helpful for this differentiation. Based on these observations, which support a possible heterogeneity in the pathogenesis and biological behavior of the different tumor types, all experts at a consensus conference and the International Society for Diseases of the Esophagus agreed that this classification should form the basis for defining, assessing, and reporting treatment of adenocarcinoma arising in the vicinity of the esophagogastric junction (6) and differentiating adenocarcinoma of the distal esophagus from the other tumor entities. This classification is now accepted worldwide, and an increasing number of publications on this topic are based on it.
Table 18.1 Comparison of patient characteristics for those with squamous cell esophageal cancer and adenocarcinoma of the distal esophagus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Prognostic Factors
The presence of distant hematogenous metastases constitutes the single most important prognostic factor in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. The median survival of such patients is in the order of 6 to 12 months irrespective of the location and histologic subtype of the primary tumor, and can not usually be prolonged significantly by any of the available therapeutic modalities.
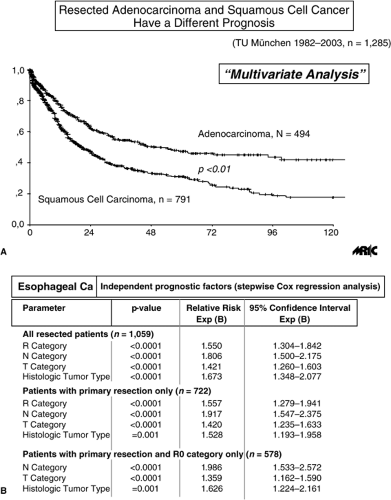
In patients without systemic metastases, a complete macroscopic and microscopic tumor resection (i.e., a R0 resection according to the guidelines of the International Union Against Cancer and the American Joint Committee on Cancer [10]) constitutes the most powerful independent prognostic factor (Fig. 18.1A, B) (11). The chance for achieving a complete tumor resection in these patients with primary surgery clearly depends on the tumor location and the pathological T (pT) category (Table 18.2).
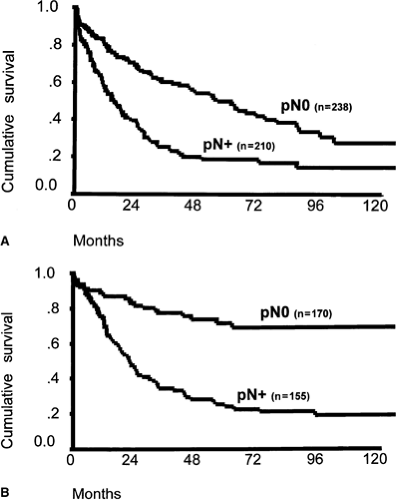
In the subgroup of patients who undergo a complete tumor resection, the lymph node status and the number of positive lymph nodes represent the major independent prognostic factors (11) (Fig. 18.2A, B). The prevalence of lymph node metastases depends on the tumor location and the pT category (Table 18.3). An independent prognostic effect of “microinvolvement” of lymph nodes that were negative by routine histologic examination has also been demonstrated for patients with squamous cell esophageal carcinoma (12,13). In primarily resected adenocarcinoma of the esophagus, the invasion of lymphatic vessels has also been shown to be an independent prognostic factor (14).
The clinical relevance of immunohistochemical detection of epithelial tumor cells in the bone marrow of patients with
esophageal cancer is still debated. At least in some of the published reports, this observation was identified as a strong predictor of early relapse and overall poor prognosis.
esophageal cancer is still debated. At least in some of the published reports, this observation was identified as a strong predictor of early relapse and overall poor prognosis.
Of the treatment-related factors, the experience of the treatment center and the surgeon performing the resection have been clearly identified as independent prognostic factors for long-term survival in patients with esophageal cancer (15,16,17,18). The amount of perioperative blood transfusion required and the postoperative morbidity appear to constitute further independent prognostic factors for duration of survival (11,19).
A clear overall survival benefit has not yet been demonstrated for extended lymphadenectomy in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. Nevertheless, several studies indicate that extended lymphadenectomy may improve survival in the subgroup of patients with a limited number of positive lymph nodes or early stages of lymphatic spread, that is, lymph node microinvolvement (20). The lymph node ratio—the ratio of positive nodes to total nodes removed—is a parameter for estimating the extent of lymph node dissection in relation to lymphatic tumor spread. A lymph node ratio of <0.2 constitutes an independent favorable prognostic factor for patients with squamous cell carcinoma and adenocarcinoma of the esophagus (21,22). The potential benefit of extended lymphadenectomy may be nullified, however, if an associated increase in postoperative morbidity occurs. A new aspect brings the so-called induction chemotherapy. In patients with complete resection, the response to induction chemotherapy is a decisive prognostic factor. Discussion regarding the therapeutic consequences of induction chemotherapy is ongoing. So far, patient-related factors (i.e., age, gender, general status) have not convincingly been shown to have an independent effect on long-term survival after complete tumor resection in patients with esophageal cancer (11).
Staging as a Prerequisite for Tailored Therapy
A tailored therapeutic approach requires exact pretherapeutic staging for selection of an adequate treatment modality. After histologic confirmation, classification, and exact topographic localization of the tumor, determination of the depth of tumor
infiltration into the organ wall (T category), the lymph node status (N category), and the presence or absence of distant metastases (M category) thus becomes essential.
infiltration into the organ wall (T category), the lymph node status (N category), and the presence or absence of distant metastases (M category) thus becomes essential.
Table 18.2 Rate of complete macroscopic and microscopic tumor resection (R0 resection by UICC/AJCC definition) in squamous cell carcinoma and adenocarcinoma of the esophagus according to pathological T (pT) category (UICC/AJCC classification) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Today, the pT category of an esophageal, esophagogastric junction, or gastric carcinoma can be predicted by endoscopic sonography with a diagnostic accuracy of approximately 85% in experienced hands. Problems still arise in the differentiation of a T2 from a T3 tumor and a T1a from a T1b tumor. The presence and extent of infiltration into neighboring organs in patients with esophageal cancer can best be assessed by computed tomography (CT) and bronchoscopy. The multislice contrast-enhanced CT scan can answer nearly all important topographic questions and is now the most decisive diagnostic procedure. Unfortunately, it has only a low accuracy to separate T1a from T1b and T2 from T3 cancer (<80%). Magnetic resonance imaging (MRI) does not add any useful information in principle but may be done if rare aortal infiltration is suspected.
Table 18.3 Prevalence of lymph node metastases in squamous cell carcinoma and adenocarcinoma of the esophagus by pathological T (pT) category (UICC/AJCC classification) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
None of the available imaging techniques (CT, MRI, endoscopic ultrasonography) can reliably predict the presence of lymph node metastases. The problem with all imaging techniques is that lymphatic spread can only be inferred by the documentation of enlarged nodes, which gives an accuracy of <70%.
Percutaneous ultrasonography of the upper abdomen, plain chest radiography, CT scanning, positron emission tomography (PET), and diagnostic laparoscopy are used to assess for distant metastases. (These staging techniques are described in other chapters.) PET/CT can give complete diagnostic information such as follows:
Topographic information that is essential for radiation and surgical treatment concepts (CT and PET)
Distant lymph nodes (LN) and distant visceral metastases (CT and PET)
Information about the tumor metabolism
By doing this, PET/CT offers a new approach to response evaluation of neoadjuvant treatment.
If neoadjuvant therapy is considered, the patient should also be evaluated for adequate liver, renal, and bone marrow function. Because preoperative radiation or combined RCT appears to increase postoperative morbidity after an esophagectomy (23), a thorough evaluation of the physiological reserve and general status is essential in these patients to make sure that they can withstand a potentially prolonged and complicated postoperative course. In the authors’ experience, a detailed risk analysis using a dedicated organ function scoring system has proved helpful in patient selection (24).
Therapeutic Options
Surgical Resection
General Principles
Most surgeons agree that complete resection (R0 resection) of the tumor and its entire lymphatic drainage offers the best chance for long-term survival in patients with esophageal cancer. In patients in whom tumor resection is incomplete (R1 or R2 resection), the procedure must be considered palliative. These patients gain no survival benefit from resection. Today, palliation of dysphagia in patients with nonresectable esophageal cancer can be achieved better and more safely by endoscopic intervention, intraluminal irradiation, external beam RT, or combined RCT. A complete macro- and microscopic tumor resection must consequently be the aim of any surgical approach to squamous cell carcinoma and adenocarcinoma of the esophagus. Palliative resections have been abandoned at most institutions.
With standardized resection and reconstruction techniques, advances in complication management, and careful patient selection, a transthoracic or transmediastinal esophagectomy with en bloc two-field lymphadenectomy can be performed with a postoperative mortality of <5%. In the authors’ experience, postoperative mortality can be decreased to <2% by application of a procedure-specific risk scoring system and exclusion of high-risk patients from surgery (24). Such results, however, can be achieved only in experienced centers with a high patient load (high-volume centers). Concentration of esophageal cancer surgery in centers with high experience and a documented history of excellence are therefore recommended.
The role and optimal extent of lymphadenectomy for esophageal cancer remains controversial. In the Western world, the benefits of lymphadenectomy for esophageal cancer so far have not been proven in large and well-designed prospective randomized trials, except in the Dutch trial for Barrett Ca. A comparison of the results from phase III trials from various centers using different strategies for lymphadenectomy, however, indicates that extended lymphadenectomy can improve the prognosis for patients with an early stage of lymphatic spread (15). In the authors’ experience, an en bloc esophagectomy with two-field lymphadenectomy (abdominal lymph node dissection and extended mediastinal lymphadenectomy) resulted in an overall 10-year survival rate of approximately 20% for patients with squamous cell carcinoma, and the survival for patients with adenocarcinoma of the esophagus was slightly better (∼35%) (21,22). The lymph node ratio constituted one of the major independent predictors of long-term survival in this analysis. The prognosis was dismal if >20% of the removed lymph nodes contained metastatic tumor on routine histologic assessment. The gain that can be achieved with lymph node dissection is thus highest for tumors at the early stage of lymphatic spread, that is, with only a limited number of positive lymph nodes. Consequently, the Dutch trial (25) was able to demonstrate a strong trend in favor of the transthoracic resection (vs. transhiatal resection). In the authors’ experience, the transthoracic resection is therefore the standard procedure for Barrett Ca.
Even more extended forms of lymphadenectomy have been reported by some centers, particularly for patients with squamous cell esophageal cancer (19,20,21,22). Although a number of retrospective series showed evidence of improved survival and a reduction of local recurrence rates after extended three-field lymphadenectomy (abdominal lymph node dissection, extended mediastinal lymphadenectomy, and cervical lymphadenectomy), more recent prospective studies indicate that this may only be the case for patients with tumors located in the proximal esophagus and for patients with less than five positive lymph nodes (26,27,28,29). Of importance is that, in most of the more recent series, extended three-field lymph node dissection was associated with a marked increase in pulmonary complications and recurrent laryngeal nerve injuries requiring tracheotomy. This limits the potential benefits of three-field lymphadenectomy. In the authors’ practice, a two-field lymphadenectomy is therefore considered standard in any potentially curative surgical approach to esophageal cancer (20).
Surgical Approach to Squamous Cell Esophageal Cancer
In patients with squamous cell esophageal cancer, a subtotal esophagectomy is usually indicated due to the frequent longitudinal submucosal lymphangiosis. The subtotal esophagectomy and reconstruction can be best performed via a right transthoracic and abdominal approach. A right transthoracic and abdominal approach is also required for adequate mediastinal and upper abdominal lymphadenectomy (two-field lymphadenectomy). At most institutions, a two-field lymphadenectomy constitutes an essential part of the procedure and comprises the following (30):
Periesophageal lymph nodes above the diaphragm and along the vena cava superior
Lymph nodes at the tracheal bifurcation
Paratracheal lymph nodes together with the nodes along the left recurrent nerve
Abdominal suprapancreatic lymphatic compartment along the celiac axis
Because of the early lymphatic spread with a risk of about 20% even in T1 tumors, lymphadenectomy is also performed in patients with T1 tumors. In the authors’ opinion, limited resection has no place in early tumor stages. Limited procedures, as proposed by some Japanese centers, are indicated only for patients with high-grade dysplasia and mucosal carcinoma. This situation is rare in Western countries.
Reconstruction after transthoracic en bloc esophagectomy is usually performed with a gastric tube and cervical anastomosis (31). A high intrathoracic anastomosis may be justified in patients who have had previous surgical procedures or radiation in the neck area, in those for whom recurrent nerve injury must definitively be avoided (i.e., singers and public speakers), or in those with a tumor located below the tracheal bifurcation.
After RCT, differentiating scars from residual tumor is frequently difficult, even during the surgical procedure. The extent of resection after neoadjuvant therapy therefore matches that of the primary surgical resection. The postoperative course after combined RCT is more severe than after neoadjuvant chemotherapy without radiation or after a primary resection. A radiation-induced compromise in immune function appears to account for this observation (32). This has prompted us to perform the reconstruction after a delay of 1 to 2 weeks following esophagectomy in patients who had neoadjuvant RCT to increase the safety of the procedure. This safety concept has resulted in a marked decrease of postoperative mortality to <5% after neoadjuvant combined RCT.
In contrast to treatment of intrathoracic squamous cell esophageal cancer, neoadjuvant treatment of squamous cell cancer of the cervical esophagus allows a limitation of the subsequent surgical resection in those who respond to preoperative therapy. The simultaneous laryngectomy that is usually required can frequently be omitted after neoadjuvant therapy, and a limited resection of the cervical esophagus with reconstruction by a free jejunal graft becomes possible. This limited procedure is associated with a markedly better quality of life than is radical esophagolaryngectomy, without compromising the long-term prognosis.
Surgical Approach to Adenocarcinoma of the Distal Esophagus
The standard procedure today for the resection of adenocarcinoma of the distal esophagus is the transhiatal esophagectomy ending with an intrathoracic anastomosis. This procedure is well proven in a prospective controlled randomized trial (25). In comparison to the transhiatal esophagectomy, the transthoracic esophagectomy allows for an adequate lymphadenectomy in the mediastinum and an en bloc resection of the tumor. The reconstruction is performed by a gastric tube with a high located intrathoracic anastomosis. In the authors’ experience, this intrathoracic anastomosis has many advantages: better swallowing function, better healing of the anastomosis, fewer paresis of the recurrent laryngeal nerve, and simplified management of complications. In case of insufficiency, this type of anastomosis can be covered easily and effectively by an endoscopically inserted stent. As a consequence of this effective complication management, the mortality is now <3% and the morbidity around 20%.
The reported surgical approaches to adenocarcinoma of the distal esophagus include abdominothoracic en bloc esophagogastrectomy, subtotal esophagectomy with resection of the proximal stomach, total gastrectomy with transhiatal resection of the distal esophagus, and limited resection of the esophagogastric junction. Since the late 1980s, the authors have performed resections in more than 1,500 patients with adenocarcinoma of the esophagogastric junction and have assessed a variety of surgical approaches (6). In patients with adenocarcinoma of the distal esophagus (i.e., AEG type I tumors), the Dutch trial could demonstrate a significant difference in long-term survival in favor of patients undergoing transthoracic resection, if the tumor was removed completely. As a consequence, the transhiatal esophagectomy is now accepted as the authors’ standard procedure.
The experience with systematic lymph node dissection in patients with adenocarcinoma of the distal esophagus shows that lymph node metastases are virtually never present in patients with tumors limited to the mucosa (pT1a about 0%) and are uncommon in patients with tumors limited to the submucosa (pT1b <20%) (Table 18.3). Data indicate that this is also true when immunohistochemical techniques are used to search for micrometastases in the lymph nodes of such patients (33). In patients with more advanced tumors, lymph node metastases occur in decreasing order of prevalence in the paracardial region, the posterior lower mediastinum, the lesser and greater curve of the stomach, along the left gastric artery toward the celiac axis, at the superior border of the pancreas along the splenic artery toward the splenic hilum, and in the area of the left adrenal gland and the left renal vein (34). Lymph node metastases in the upper mediastinum or cervical region occur only in patients with locally advanced adenocarcinoma who also have numerous positive locoregional nodes.
Given this pattern of lymphatic spread, an extended lymph node dissection in patients with adenocarcinoma of the distal esophagus should include the removal of lymph nodes along the splenic artery, at the splenic hilus, and along the left renal vein behind the pancreas. To perform this retroperitoneal lymph node dissection, a left-sided pancreatic resection with splenectomy should be avoided because this procedure is associated with a substantial number of septic complications due to pancreatic fistula and abscess formation. Although pancreas-preserving splenectomy allows a similar clearance of lymph nodes in this area without the risk of pancreatic fistula, splenectomy itself may result in significant morbidity. Because postoperative complications independently influence long-term survival, safe resection and reconstruction techniques are essential. Consequently, the potential benefits of a more extensive lymph node dissection achieved with splenectomy may be nullified by the associated morbidity. Splenectomy is therefore only justified in patients with frank lymph node metastases or infiltration of the splenic hilum.
The morbidity associated with extended total gastrectomy or esophagectomy and the compromised quality of life after these procedures have in recent years stimulated efforts to assess more limited forms of resection for adenocarcinoma of the distal esophagus. Based on the virtual absence of lymph node metastases and micrometastases in patients with tumors limited to the mucosa and the low prevalence and number of lymph node metastases found in patients with tumors extending to the submucosa, a limited resection of the distal esophagus, cardia, and proximal stomach has been evaluated in such patients (33). To avoid postoperative reflux, reconstruction is performed by interposition of a pedicled jejunal segment. In the authors’ experience of performing more than 100 such procedures for tumors staged as uT1 on endoscopic ultrasonography, a complete resection (R0) could be achieved in all instances. No evidence of lymph node metastases or micrometastases was found in a mean of 20 removed nodes per patient. So far, in a 2-year follow-up, no recurrences or deaths have occurred. Quality-of-life assessment showed no evidence of gastroesophageal reflux and good to excellent swallowing function in >90% of the patients (33). Similar encouraging data with limited resection in patients with early tumors at the esophagogastric junction, particularly those in which the vagus nerve can be preserved during the resection, are also reported by several Japanese authors.
Endoscopic Mucosa Resection
The new technology of endoscopic mucosa resection offers an even more limited approach to early tumors of the distal esophagus (35). Because a lymphadenectomy is not possible with this technique, endoscopic mucosa resection can only be recommended for patients with pT1a tumors. The frequent multicentric tumor growth; the inaccuracy of current preoperative staging modalities, including high-frequency endoscopic ultrasonography, in differentiating mucosal from submucosal tumors; and the persistence of precancerous lesions (i.e., Barrett esophagus) currently restrict the clinical application of trial protocols.
Combined Modality Treatment
Despite the remarkable progress that has been documented over the past decades in the surgical treatment of esophageal cancer, prospects for long-term survival of these patients, even after complete resection, are still dismal. This is because more than two-thirds of patients present with tumors that have grown beyond the esophageal wall, such as tumors invading the adventitia (T3) or adjacent structures (T4). A complete resection can be achieved in only a minority of these patients (Table 18.2). Furthermore, esophageal carcinoma metastasizes early during the course of the disease. Autopsy studies demonstrate that a large percentage of patients already have systemic disease at the time of presentation (36). Lymphatic spread is common in tumors that extend beyond the mucosal layers (Table 18.3). Finally, the close anatomical relationship of the proximal esophagus to the tracheobronchial tree prohibits extensive resection in patients with esophageal tumors located at or above the level of the tracheal bifurcation. Thus, primary surgical resection is reasonable only in those patients for whom preoperative staging indicates that a R0 resection can be achieved with a high degree of certainty.
Consequently, multidisciplinary approaches using adjuvant, neoadjuvant, or additive therapeutic methods have received
increasing attention. Targets for the additional treatment are the local extraesophageal tumor growth in T3 and T4 tumors (tumor bed) and occult locoregional and distant micrometastases. Modalities include radiotherapy, chemotherapy, or combined chemoradiation.
increasing attention. Targets for the additional treatment are the local extraesophageal tumor growth in T3 and T4 tumors (tumor bed) and occult locoregional and distant micrometastases. Modalities include radiotherapy, chemotherapy, or combined chemoradiation.
Despite numerous phase II and phase III trials, the role of multimodal therapy in the treatment of esophageal cancer is still under discussion. This is because most studies are not comparable. Some studies show remarkable shortcomings in study design, others lack adequate pretherapeutic staging, and yet others use confusing terminology. The major limitations of most available studies are (2,37) as follows:
Inaccurate staging and patient stratification according to prognostic factors
Major differences in the staging systems (American Joint Committee on Cancer/International Union Against Cancer) applied before 1997
Different definition of locoregional and locally advanced disease
Imprecise information concerning the quality and extent of surgical resection and lymphadenectomy
Variations in the definition of “curative resection”: curative according to the surgeon (who misses microscopic residual disease in up to 20% of the patients) versus macroscopic and microscopic complete resection (R0) according to the surgeon and pathologist
Differences in the quality of the pathological-histologic workup of the resected specimen and pathological reporting
Failure to discriminate squamous cell carcinoma and adenocarcinoma of the esophagus
A broad spectrum of variably defined radiation schedules
Each factor listed here can influence the prognosis of a patient to a greater extent than the potentially beneficial multimodal therapy. This must be kept in mind when analyzing the published reports.
Postoperative Adjuvant Treatment
By definition, adjuvant therapy is postoperative treatment after macroscopic and microscopic complete resection (R0), as far as surgeon and pathologist can exclude micrometastases beyond the resection margins. The aim is to eradicate left tumor cells beyond resection margin and occult regional and distant micrometastases to prevent or delay locoregional and distant recurrence. An advantage of adjuvant treatment is that pathological evaluation of the resected specimen and intraoperative staging provide definitive pretreatment information. The surgeon can define areas of risk and thus focus postoperative therapy. However, one major shortcoming of adjuvant therapy in contrast to neoadjuvant therapy is that it cannot lead to tumor shrinkage, and therefore, it will not contribute to a higher potentially curative R0 resection rate. In the light of the limited chances for R0 resection in locally advanced esophageal cancer (Table 18.2), this consideration constitutes a strict hypothesis against the adjuvant approach in this setting.
Even after a complete resection, patients with pT3 and pT4 or pN-positive tumors are at a high risk for recurrence. For example, patients with disease classified as pT3 N0 have a risk of at least 60% of dying from local or distant tumor recurrence within 5 years after the operation. Such patients are therefore potential candidates for postoperative adjuvant treatment. Only a minority of eligible patients, however, tolerate an intensive postoperative treatment protocol. This is due to a generally reduced performance status after esophagectomy, which is accompanied by a potentially increased toxicity of the treatment with subsequent poor compliance. Furthermore, adjuvant therapy is often initiated after a long delay due to postoperative complications. From an anatomical and physiological point of view, postoperative therapy is also hindered by tumor cell entrapment, hypo-oxygenation, and altered blood supply in the areas of interest.
Postoperative Radiotherapy
Postoperative changes in the anatomy may result in the presence of larger areas of uninvolved tissue (e.g., the organ used for reconstruction) in the irradiated volume. This leads to an increase in morbidity. The assumed decreased oxygenation of residual tumor cells results in decreased radiosensitivity and possibly in the selection of tumor clones resistant to cytotoxic therapy (38).
The rationale for using adjuvant radiation is based on the pattern of failure after a complete resection; only a few surgical series have reported such data. The rates of local failure in the surgical control arms of two randomized trials of preoperative radiation therapy were 12% and 67%, respectively (39,40). Local failure rates in the surgical control arm of the randomized postoperative radiation therapy trial of Teniere et al. were 35% for patients with negative locoregional lymph nodes and 38% for those with positive nodes (41). Although the majority of patients with esophageal cancer die of distant metastasis, the incidence of local failure after surgery alone is high enough to examine the use of adjuvant radiation therapy.
Nonrandomized trials have reported encouraging results with postoperative radiation therapy. In a study by Kasai et al., patients with lymph node–negative disease had a 5-year survival rate of 88% (42). Yamamoto et al. reported a 2-year local control rate of 94% in node-negative patients (43).
Data on five randomized trials comparing postoperative radiotherapy and resection with resection alone are reported. Only three trials suggested that postoperative radiation may actually decrease the local failure rate (43,44,45), but at the expense of significant morbidity in two trials, with one single trial even demonstrating an adverse but nonsignificant effect of additional radiotherapy on overall survival, perhaps due to hypofractionated schedule (single dose of 3.5 Gy) (44). None of these trials observed an overall survival benefit for patients receiving adjuvant radiation; this was also confirmed by a negative meta-analysis (46).
Teniere et al. reported on 221 patients with squamous cell esophageal cancer randomly assigned to receive surgery alone or surgery plus postoperative radiation therapy (45–55 Gy at 1.8 Gy per fraction). After a minimum follow-up of 3 years, adjuvant radiation was not found to prolong survival (41) (Table 18.4). These data were confirmed by further clinical trials with no significant difference in 2-, 3-, or 5-year overall survival even with large total doses of up to 60 Gy (45,47), although the latter was conducted in nearly 500 patients. A second trial by Fok et al. included patients with squamous cell carcinoma and adenocarcinoma of the esophagus (44). Patients with a complete or palliative resection were included in this trial. Even in this study with an extraordinary risk of postoperative local tumor progression after incomplete tumor resection, the addition of postoperative radiation therapy did not significantly decrease local (31%–15%) or distant failure, or improve median survival.
Adjuvant radiation therapy is sometimes recommended for patients with positive locoregional lymph nodes. Although the data from Teniere et al. suggest that postoperative radiation therapy may reduce local failures, the benefit was limited to node-negative patients. In this subset, adjuvant radiation therapy decreased the local failure rate from 35% to 10%.
That means there is no indication for postoperative radiation after complete or incomplete resection. Side effects are increased by postoperative radiotherapy, which may be due to high total doses in some trials (45,47) and lack of modern conformal radiotherapy with optimal sparing of critical organs as lung in all studies published in the 1990s from trials started in the 1980s. Therefore, postoperative
radiotherapy might be offered if the risk of distant metastases is estimated comparatively low, and the patient has recovered rapidly and completely after resection. It is an individual decision that should be based on multidisciplinary discussion and the decision of an informed patient. The use of conformal radiotherapy is a prerequisite then.
radiotherapy might be offered if the risk of distant metastases is estimated comparatively low, and the patient has recovered rapidly and completely after resection. It is an individual decision that should be based on multidisciplinary discussion and the decision of an informed patient. The use of conformal radiotherapy is a prerequisite then.
Table 18.4 Postoperative radiotherapy and survival for esophageal carcinoma after complete resection: randomized phase III trials | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Intraoperative radiotherapy (IORT) constitutes a special form of adjuvant radiotherapy. Different IORT techniques and doses have been examined in patients with esophageal carcinoma. Single IORT doses of 25 Gy caused tracheal damage in almost 30% of patients (48). Because patients receiving IORT were not compared with untreated controls, the effect on local recurrence rates and long-term survival in esophageal cancer remains unknown.
In summary, although the limited data available suggest that the use of adjuvant radiation therapy in esophageal cancer may decrease local failure in node-negative patients, it has no proven impact on overall survival (46). The only established role for postoperative radiation therapy might be in patients who have positive tumor margins after resection. Based on the positive survival results from combined modality therapy trials, postoperative radiation should be combined with systemic chemotherapy, if used at all.
Table 18.5 Postoperative chemotherapy for esophageal carcinoma: prospective, randomized trials | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Postoperative Chemotherapy
The use of postoperative chemotherapy has been assessed in four randomized trials, with three trials comparing it with surgery alone. A French multicentric study investigated the effect of cisplatin-based combination therapy (cisplatin and 5-fluorouracil [5-FU]) after complete resection versus surgery alone. No difference in survival was seen between the two groups (49). Significantly more patients in the treated group had hematologic, neurologic, or renal complications. In a randomized trial, the Japanese Esophageal Oncology Group compared postoperative chemotherapy with cisplatin and vindesine to surgery alone in 205 patients with squamous cell esophageal cancer (50). They reported no significant differences in survival between groups, even after stratification for lymph node status. A newer Japanese trial randomized 402 patients to surgery alone or surgery and chemotherapy (two courses of cisplatin and 5-FU). Again, this trial revealed no statistical significant difference in survival and diseasefree survival between the two groups, but the risk reduction by postoperative chemotherapy was remarkable for patients with lymph node metastases, with an absolute improvement in 5-year overall survival of 14% (51) (Table 18.5). However, there was stratification only based on resection margin and not on nodal status, which means that this study does
not sufficiently justify postoperative chemotherapy in clinical routine.
not sufficiently justify postoperative chemotherapy in clinical routine.
All studies that investigated chemotherapy using a “sandwich method” (i.e., preoperative and postoperative chemotherapy) reported failure. The projected postoperative chemotherapy could not be administered due to unacceptable toxicity after the operation. In a study by Heath et al., the combination of cisplatin and paclitaxel to be given postoperatively could be administered to <50% of the patients due to unacceptable myelosuppression and fatigue (52).
Postoperative Radiochemotherapy. Postoperative chemoradiation has so far been studied in only one phase II trial (53), which does not allow any conclusions.
Based on these data, postoperative adjuvant chemotherapy after complete resection of esophageal cancer has so far shown no efficacy in prolonging diseasefree and overall survival, and consequently, has no established role outside clinical trials. The potential reduction of local recurrences after postoperative radiation therapy did not result in a prolonged overall survival. Neither is there evidence that supports the idea of postoperative radio-, chemo- or RCT even in R1–2 resection. However, in a single situation with informed consent of the patient, it might be justifiable to offer an additional treatment to patients after incomplete tumor resection, to avoid an early local tumor progression. The high toxicity after previous esophagectomy (hematotoxicity CTC°III–IV >20%, gastrointestinal toxicity CTC°III–IV >30%) should be taken into account, and the patient should be offered an optimal supportive care program.
Preoperative Treatment
Three major approaches to preoperative combined modality therapy have been explored in patients with locoregional or advanced esophageal cancer since the 1970s: preoperative radiotherapy, chemotherapy, and chemoradiation. After phase II trials demonstrated the safety of these techniques, random assignment studies were performed in an effort to improve the chances for a complete tumor resection and to prolong overall survival.
Several theoretical and clinical factors favor the use of preoperative therapy over postoperative therapy (2,54):
Blood and lymph vessels are undamaged, which provides an effective drug concentration in the problem areas around the tumor, and tumor oxygenation and radiosensitivity are preserved.
Table 18.6 Preoperative radiotherapy for patients with esophageal cancer: randomized phase III trials
Study
Protocol
Histology
No. patients
Resection rate (%)
Mortality (%)
Median Survival (mo)
Survival rate (%)
p
Value
2 y
5 y
Launois et al. (56)
Surgery
SCC
47
70
23
12
35
12
NS
40 Gy
62
76
23
10
22
10
Gignoux et al. (40)
Surgery
SCC
106
82
18
45
28
9
NS
35 Gy
102
74
24
48
26
10
Wang et al. (57)
Surgery
SCC
102
85
6
NA
NA
30
NS
40 Gy
104
93
5
—
—
35
Arnott et al. (58)
Surgery
SCC
86
72
13
8
30
17
NS
20 Gy
90
74
15
8
25
9
Fok et al. (59)
Surgery
SCC
39
NA
8
22
40
16
NS
35–53 Gy
40
37
11
35
10
SCC, squamous cell carcinoma; NS, not significant; NA, not available.
The performance status of the patient is better than it is postoperatively, which allows the administration of a more aggressive cisplatin-based combination chemotherapy.
The performance status of responding patients is improved preoperatively.
The tumor is “downsized” (or, better, “downshrunk”); thus, the possibility for a complete resection is improved.
Systemic micrometastases are eliminated early.
Preoperative therapy may devitalize tumor cells and minimize the risk of intraoperative spillage and seeding of viable tumor cells.
The efficacy of preoperative radiation, chemotherapy, or both can be studied histopathologically in the resected specimen.
Patients who may not tolerate aggressive combined preoperative treatment and resection can be detected before resection to avoid high perioperative mortality.
Patients with minor tumor response and severe toxicity of preoperative treatment may be omitted for resection to avoid high mortality.
Prerequisite for neoadjuvant treatment is an exact pretherapeutic staging. This is lacking in most reported trials. More than 90% of studies published so far use only on endoscopic or radiographic staging criteria. Endoscopic ultrasonography is used in <20% of hospitals dealing with esophageal cancer patients, and the overall availability of endoscopic ultrasonography in all hospitals in the United States does not exceed 5% (55).
Preoperative Radiotherapy
Preoperative radiotherapy (20–55 Gy) was studied in seven randomized trials, starting as early as 1968 (Table 18.6), with five trials against surgery alone, one trial within a four-arm setting against surgery alone and against postoperative radiotherapy, and one trial against postoperative radiotherapy (46). Some studies observed that resectability was slightly higher in patients treated with preoperative radiotherapy than in untreated patients (56,57,58,59), whereas others reported the opposite. In two studies, the treatment-related mortality rate of patients receiving preoperative radiotherapy was higher than that of patients treated by esophageal resection alone (40,44), but both trials used high single doses (2.4–3.3 Gy) known to disproportionately
increase side effects (59). No survival benefit compared to primary resection has been reported from any single randomized trial; some studies even recorded a slight reduction in overall survival after preoperative radiotherapy. However, an analysis by the Cochrane Collaboration found an overall reduction of 11% in the risk of death, making an absolute survival benefit of 3% at 2 years and 4% at 5 years, respectively, with a borderline significance (p = 0.062) in pooled data of five properly designed randomized trials including 1,147 patients with a median follow-up of 9 years. The studies used for the meta-analysis mostly included patients with squamous cell cancer, making it impossible to give any advice on treatment of adenocarcinoma of the lower esophagus (AEG I). However, due to the borderline significance, preoperative radiotherapy is not recommended as standard procedure even in squamous cell carcinoma of the oesophagus (60). According to a meta-analysis by Arnott et al., inclusion of more than 3,000 patients would be necessary to perform a valid study to prove the benefits of preoperative radiotherapy (61).
increase side effects (59). No survival benefit compared to primary resection has been reported from any single randomized trial; some studies even recorded a slight reduction in overall survival after preoperative radiotherapy. However, an analysis by the Cochrane Collaboration found an overall reduction of 11% in the risk of death, making an absolute survival benefit of 3% at 2 years and 4% at 5 years, respectively, with a borderline significance (p = 0.062) in pooled data of five properly designed randomized trials including 1,147 patients with a median follow-up of 9 years. The studies used for the meta-analysis mostly included patients with squamous cell cancer, making it impossible to give any advice on treatment of adenocarcinoma of the lower esophagus (AEG I). However, due to the borderline significance, preoperative radiotherapy is not recommended as standard procedure even in squamous cell carcinoma of the oesophagus (60). According to a meta-analysis by Arnott et al., inclusion of more than 3,000 patients would be necessary to perform a valid study to prove the benefits of preoperative radiotherapy (61).
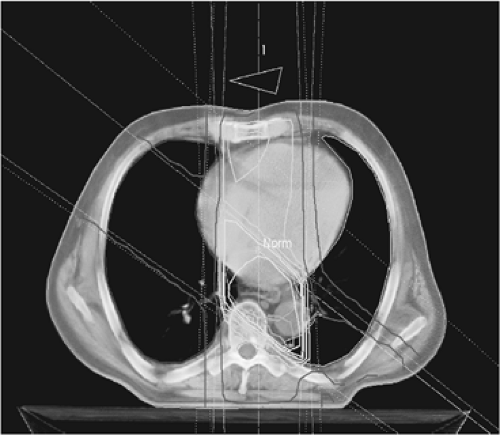 FIGURE 18.3. Example of three-dimensional conformational planning of radiotherapy for esophageal cancer. The isodose distribution is shown. |
Controversy still exists regarding fractionation (conventional fractionation vs. hyperfractionation or hyperfractionation and acceleration), dosage, target volume of radiotherapy, and timing of surgery after radiation. Because of recent advances in delivery of radiation, additional studies are required to define the role of preoperative radiation as a single modality or combined with chemotherapy. Innovative radiation approaches are under study in an attempt to maximize tumor cell damage while sparing normal tissue.
The use of three-dimensional conformational radiation planning is essential, which may allow higher doses to be delivered with less morbidity (Fig. 18.3). The use of hyperfractionated schedules allows a better repair of sublethal damage in critical organs between two smaller fractions, and the combination of accelerated fractionation with reduction of overall treatment time may overcome relative radioresistance of tumor cells.
Preoperative Chemotherapy
The impact of neoadjuvant chemotherapy on the prognosis for patients with esophageal cancer can be assessed only if patients with resectable tumors and those with locally advanced and irresectable tumors are evaluated separately. Resectable tumors include T1 and T2 categories, whereas T3 and T4 and N+ categories are considered locally advanced tumors. At least 30% of this latter group is not completely resectable (R0). Unfortunately, due to inadequate staging procedures, a lack of multidisciplinary assessment, and inconsistent definitions in clinical trials, the majority of studies does not clearly separate these two distinct clinical situations.
Phase II studies involving patients with potentially resectable tumors
By far, the majority of studies of preoperative chemotherapy in patients with potentially resectable tumors have been uncontrolled phase II trials. The first cisplatin-based combination therapy study was initiated by Kelsen’s group more than 25 years ago (62). In subsequent trials, higher response rates were observed with a combination of cisplatin, bleomycin sulfate, and vindesine (DBV, a “first-generation” chemotherapy regimen) (63,64,65,66). The rate of pathological complete responses, however, was disappointingly low (approximately 5%). Because of a significant number of postoperative deaths, which might be attributed to the potential pulmonary toxicity of bleomycin, this substance was omitted in later trials and replaced by drugs that have shown some single-agent activity against esophageal cancer. The overall results were not changed significantly.
The combination of cisplatin and 5-FU given by continuous infusion (“second-generation” combination chemotherapy) has been studied extensively in preoperative chemotherapy trials in squamous cell and adenocarcinoma of the esophagus (67,68,69,70,71,72,73,74,75,76,77). Major responses have been observed in 42% to 66% of patients, with pathologically determined complete response rates of 7% to 11%. Resectability after a preoperative regimen of cisplatin and 5-FU has ranged from 38% to 94%. Toxicity has been tolerable. Chemotherapy-related deaths were rare, and operative mortality did not seem to be increased in these studies. Next, the biomodulation of 5-FU–containing combinations by addition of leucovorin calcium (68) or interferon-α (77) or both was investigated in the “third-generation” chemotherapy protocols. The response and resectability rates, postoperative mortality, and survival reported in these trials were comparable to those with cisplatin and 5-FU alone. The toxicity, however, appeared to be higher.
Recently, the taxanes (paclitaxel and docetaxel) and irinotecan hydrochloride in combination with cisplatin (“fourth-generation” chemotherapy regimen) were introduced for the preoperative treatment of esophageal cancer. As yet, the experience is limited but promising (78,79,80).
Overall, the use of preoperative chemotherapy in patients with localized esophageal cancer is feasible and does not appear to increase postoperative morbidity and mortality. Major responses were achieved with cisplatin-based combinations in 41% to 69% of patients. Pathologically determined complete responses were reported in less than 10% of patients. Although the percentage of distant treatment failures appeared to decrease, locoregional failures remained constant with local recurrences of 30% to 40%, even after complete resection. Median overall survival times have been reported to be between 12 and 24 months, and survival rates are 40% at 2 years after resection. The phase II studies indicated that the administration of preoperative chemotherapy is tolerable and without demonstrable adverse effects on survival outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree