Esophageal Cancer: Anatomy and Staging
Richard P.M. Koehler
Frank C. Detterbeck
David A. Dean
The surgical treatment of esophageal cancer requires today’s surgeons to not only have a thorough knowledge of anatomy and surgical techniques, but also to understand and interpret the medical literature in order to efficiently work up and appropriately stage patients. Accurate staging is essential to defining prognosis, selecting appropriate treatment, and describing patient populations so studies can be compared. As is the case with most malignancies, staging of esophageal cancer uses the TNM (Tumor invasion, Nodal involvement, Metastatic disease) classification as defined by the American Joint Commission on Cancer (AJCC) (1).
Anatomy
Although it plays no role in digestive, absorptive, or endocrine functions, the esophagus is an important part of the gastrointestinal system. It serves as a conduit for solids and liquids from the oropharynx to the stomach as it traverses the posterior mediastinum from the lower neck into the upper abdomen. Despite having distinct layers (mucosa, submucosa, and muscularis propria), the esophagus lacks both mesentery and serosal layers. The mucosal layer is comprised of nonkeratinized squamous epithelium, basement membrane, lamina propria, and muscularis mucosa. The submucosal (or strength) layer of the esophagus contains connective tissue, blood vessels, lymphatics, and submucosal glands. The muscularis propria allows for the propulsive abilities of the esophagus, and is comprised of an inner circular muscle layer and an outer longitudinal oriented muscle layer.
The blood supply to the cervical esophagus arises from branches of the superior and inferior thyroid arteries in the neck, while the upper and middle thoracic esophagus receives blood from bronchial branches. The blood supply to the lower thoracic esophagus comes directly from the aorta, which are the only “true” dedicated esophageal arterial branches. The lower thoracic and abdominal esophagus receives blood supply from branches of the left gastric and splenic arteries. Venous drainage is accomplished by an extensive submucosal venous plexus, which ultimately drains into inferior thyroid, brachiocephalic, azygous, hemiazygous, and left gastric and splenic veins. Mucosal lymphatics drain directly into a rich submucosal plexus that spans the entire length of the esophagus, accounting for the often rapid and early dissemination seen in many esophageal cancers. Innervation of esophagus arises from both sympathetic and parasympathetic fibers. Vagal parasympathetics supply motor input to the muscularis propria, whereas secretomotor provides input to the submucosal glands. Sympathetic innervation, which arises from both the sympathetic chain and the celiac plexus, causes constriction of the esophageal sphincters and blood vessels and increases parastatic and glandular activity.
Definition of Staging
The stage of esophageal cancer can be defined in the context of several distinct clinical situations. The pathological stage, which is generally accepted as the gold standard, is the stage as evaluated by the pathologist after examining all tissue sampled during the course of surgical resection. At the time patients are evaluated and decisions regarding the treatment approach are made, only clinical staging is possible. Clinical staging is the stage determined from all available information, including invasive techniques, before any treatment (e.g., surgery). Finally, in the context of induction chemotherapy or radiotherapy, consideration of the stage after completion of the induction treatment, often referred to as restaging, is useful. Pathological stage, clinical stage, and restaging after initial treatment are denoted by the prefixes p, c, or y (e.g., pI or cT3N1M0). Clarity about which type of staging is being referred to is important because the implication of a particular stage can be different in each situation.
The pathological stage is usually accepted as the most accurate representation of the true stage of esophageal cancer, at least as far as intrathoracic or intraabdominal disease is concerned. The issue with regard to clinical staging or restaging after treatment is how reliably this stage correlates with the ultimate pathological stage. That is the focus of this chapter. The questions of particular interest to clinicians working with patients with esophageal cancer are regarding accurate determination of T0 to T2 versus T3 versus T4 tumors, identification of nodal involvement, and the presence of metastatic disease.
Statistics
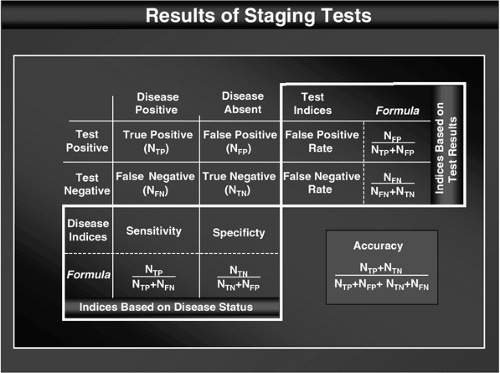
The reliability of tests used in staging is usually measured with indices such as sensitivity, specificity, false-positive (FP) rate, false-negative (FN) rate, and accuracy. Appropriate interpretation of these indices requires a thorough understanding of these parameters, how they are calculated, their inherent limitations, and how factors such as prevalence affect them. These indices are often misunderstood, as evidenced by the frequency with which people use the wrong parameter when interpreting or presenting clinical data (e.g., using the sensitivity rather than the FN rate in interpreting a negative result in a patient). To avoid confusion, the definitions of these parameters are provided in Figure 17.1.
Sensitivity and specificity are useful in selecting a test to be performed in a population of patients, but these parameters are limited because they pertain to theoretical populations, all members of which either have or do not have the condition in question. In contrast, FP and FN rates allow interpretation of the test result in individual patients, which permits a more refined estimate of the true disease status. These latter parameters are generally of more practical use to the clinician. The FP and FN rates are often expressed a bit less concretely as the positive and negative predictive values. Accuracy represents the fraction of “correct results” and is strongly affected by disease prevalence. It represents a combination of these other measures, but with such a loss of detail that interpretation of the results is rendered nearly impossible. For example, a test may be highly “accurate” but have a sensitivity of zero if the specificity is high and the prevalence is low. Therefore, although accuracy may satisfy a desire to express the reliability as a single parameter, it has virtually no practical application and is of limited value.
FP and FN rates are affected by the overall prevalence of the condition in the entire population. This probably makes these parameters less appealing to statisticians and may explain why more emphasis has been placed on sensitivity and specificity in the medical literature. The prevalence has little effect on FP and FN rates, however, unless extremes of prevalence are encountered (i.e., <10% or >90% prevalence), at least in the case of tests that have a reasonable sensitivity and specificity (i.e., >80%). Therefore, this chapter focuses on FP and FN rates but attempts to exclude data from studies that had a prevalence of <10% or >90%.
TNM Definitions
Esophageal cancer is usually classified according to the TNM system. Definitions of these TNM classes are based on pathological findings and are shown in Table 17.1. Tis and T1 (invasion into the submucosa but not muscle) tumors represent minimal invasion of the esophageal wall. T2 tumors invade into, but not through, the muscle into the periesophageal tissue; the latter type of invasion is the definition of T3 tumors. T4 tumors invade adjacent structures such as the aorta, trachea, or pericardium (Fig. 17.2). Nodal status (N) is based solely on the presence (N1) or absence (N0) of involvement of periesophageal lymph nodes by standard histologic evaluation. M1 disease represents distant metastases. The latest revision of the TNM system defines positive lymph nodes in cervical and celiac nodal regions as distant metastatic disease (M1a) and solid organ metastatic disease as M1b. Table 17.2 shows the TNM categories that comprise the stage groups in esophageal carcinoma (2).
Although the pathological definition of esophageal cancer has been clearly defined by the AJCC, the definitions used in clinical staging are slightly different, at least in the case of computed tomography (CT) definition of the T status. The criteria commonly used for CT and endoscopic ultrasound (EUS) are shown in Table 17.1. The clinically important issues in clinical staging are the differentiation between T1 and T2, T3, and T4 tumors; identification of N1 involvement; and recognition of the presence of M1 disease.
Diagnostic Modalities
Computed Tomography
A staging CT for esophageal carcinoma includes the supraclavicular area; the lungs and mediastinum; the liver, adrenal glands, and gastrohepatic ligament; and the celiac nodal areas. CT is often used as an initial study for the assessment of T status and M status, but it is quite unreliable in determining N status. The normal thoracic esophagus varies in the degree of
wall thickness, depending on distention with oral contrast, but measurements of >5 mm are routinely considered abnormal (3). Small primary tumors of the esophagus may be difficult to see by CT. Furthermore, CT cannot discriminate between the histologic layers of the wall of the esophagus, making it difficult to differentiate between T stages. Tumor invasion is suggested when normal fat planes are lost between tumor and adjacent structures or when a mass effect is present (4,5,6). CT also occasionally plays a role in guiding a fine-needle aspiration (FNA) biopsy.
wall thickness, depending on distention with oral contrast, but measurements of >5 mm are routinely considered abnormal (3). Small primary tumors of the esophagus may be difficult to see by CT. Furthermore, CT cannot discriminate between the histologic layers of the wall of the esophagus, making it difficult to differentiate between T stages. Tumor invasion is suggested when normal fat planes are lost between tumor and adjacent structures or when a mass effect is present (4,5,6). CT also occasionally plays a role in guiding a fine-needle aspiration (FNA) biopsy.
Table 17.1 Definition of TNM for carcinoma of the esophagus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 FIGURE 17.2. Schematic of tumor (T) staging. Source: Reprinted from Rice TW. Clinical staging of esophageal carcinoma. Chest Surg Clin N Am 2000;10:473 , with permission of Elsevier. |
Table 17.2 Stage groupings for carcinoma of the esophagus | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Magnetic Resonance Imaging
Endoscopic Ultrasound
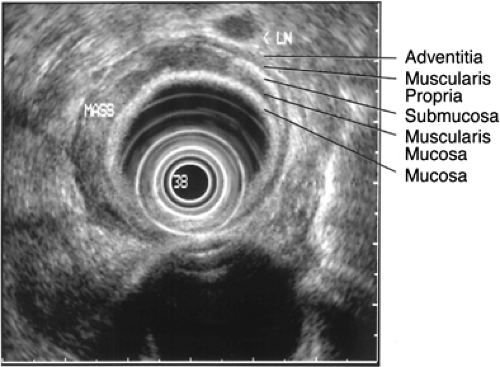
EUS uses high-frequency (5- to 20-MHz) transducers incorporated into the tip of a flexible endoscope or in a separate fiber-optic instrument passed through a normal scope to create ultrasonic images. A 7.5-MHz transducer provides reasonable resolution to a depth of 5 to 7 cm, whereas a 12-MHz transducer provides resolution to a depth 2 to 3 cm with spatial resolution of 2 mm. Ultrasound of a normal esophagus produces an image consisting of five layers (Fig. 17.3). The innermost first layer is hyperechoic (white) and represents the mucosal interface. The second layer is hypoechoic (black) and corresponds to the muscularis mucosa. The third layer is hyperechoic and corresponds to the submucosa. The fourth layer (hypoechoic) corresponds to the muscularis propria and the fifth layer (hyperechoic) to the adventitial interface (Table 17.1). EUS provides not only reliable T staging but can also be used to assess regional lymph nodes. Criteria for abnormal pathology include lymph node shape, border characteristics, and central echogenicity (10). EUS-directed FNA is a useful modality for obtaining tissue samples of suspicious periesophageal lymph nodes during ultrasound staging procedures.
Positron Emission Tomography
Positron emission tomography (PET) is an imaging modality that provides an assessment based on differences in the cellular metabolism as opposed to the anatomical abnormalities that serve as the basis for CT, MRI, and EUS. Most commonly, this involves administration of 2-deoxyglucose labeled with fluorine 18 (FDG), a radionucleotide that decays by positron emission. FDG is taken up into cells in proportion to glucose utilization, and as such, tumor cells, which have high energy requirements, take up large amounts of FDG. In these tumor cells, FDG is phosphorylated but then cannot be rapidly metabolized because many types of tumor cells have very low levels of glucose-6-phosphatase. Normal cells, in contrast, with higher levels of glucose-6-phosphatase can more rapidly dephosphorylate and thereby metabolize FDG-6-phosphate; this provides the physiological basis of the PET scan. Simply put, this means that FDG is in essence trapped within tumor cells, and thus, tumor cells can be detected because of higher rates of positron emission as the FDG decays (11). A limitation of PET scanning is the poor anatomical definition, particularly of the early generation scanners, so that separation of the primary tumor from adjacent lymph nodes can be difficult. In an attempt to improve on the lack of precise anatomical detail, many modern PET scanners have incorporated CT imaging capabilities, creating fusion PET/CT images. This allows for the simultaneous acquisition of both PET and CT data, and with real-time comparison and integration of the images, it is now possible to interpret PET data with more anatomical precision.
Other Noninvasive Tests
Few other imaging studies or laboratory tests are warranted in the staging of patients with esophageal cancer. A chest radiograph is usually obtained but is of limited value because it is normal in >70% of patients with esophageal cancer (16). CT imaging and PET scans both provide a better overall assessment for metastatic disease, and unlike chest radiographs, are not limited to the chest. Screening blood tests are neither sensitive nor specific for determining possible liver metastases unless liver involvement is massive. Although a barium swallow is routinely performed in the initial workup of a patient with dysphagia, it provides only a limited amount of information regarding the degree of luminal narrowing and the location and length of the lesion and provides no staging information. With the availability of endoscopy and CT, a barium study has become less important, and most clinical staging issues are better addressed by CT or EUS, once the diagnosis of esophageal cancer has been made.
Invasive Modalities
Invasive staging consists of surgical evaluation of the primary tumor and the lymph nodes, as well as surveying the liver for possible metastases. Invasive staging can be justified by the fact that several groups have demonstrated nodal involvement
in remote areas at the time of resection that was not appreciated by conventional preoperative noninvasive staging studies. Akiyama et al. found that 32% of patients with upper esophageal tumors had disease in abdominal lymph nodes (17). Two additional studies evaluating the three-field lymphadenectomy demonstrated positive cervical lymph nodes in 20% to 30% of patients with thoracic and abdominal esophageal carcinoma (18,19). Furthermore, current techniques in molecular biology and immunohistochemistry allow a more sensitive evaluation of nodes for malignant involvement than was previously possible by routine histologic assessment (20,21,22).
in remote areas at the time of resection that was not appreciated by conventional preoperative noninvasive staging studies. Akiyama et al. found that 32% of patients with upper esophageal tumors had disease in abdominal lymph nodes (17). Two additional studies evaluating the three-field lymphadenectomy demonstrated positive cervical lymph nodes in 20% to 30% of patients with thoracic and abdominal esophageal carcinoma (18,19). Furthermore, current techniques in molecular biology and immunohistochemistry allow a more sensitive evaluation of nodes for malignant involvement than was previously possible by routine histologic assessment (20,21,22).
In 1977, Murray et al. described preoperative surgical staging of esophageal cancer with mediastinoscopy and a minilaparotomy in a prospective study involving 30 patients (23). Celiac nodal involvement was found in 53% and mediastinal involvement in 23%, although these authors believed that this staging technique did not optimally stage the chest. Krasna et al. examined the utility of combined preoperative thoracoscopy and laparoscopy in a prospective multiinstitutional phase II trial (CALGB 9380) (24). This study established that thoracoscopy and laparoscopy for staging of esophageal cancer was safe and feasible. Later, Krasna et al. compared thoracoscopic and laparoscopic staging with conventional staging; they found that invasive staging provided more accurate information about local tumor invasion, regional lymph node involvement, and distant metastases (25). Clements et al. confirmed the utility of laparoscopy as part of a preoperative staging algorithm for distal esophageal and proximal gastric cancers. By using this invasive staging technique, they were able to prevent unnecessary laparotomy in 18% of patients who had been deemed “resectable” by conventional imaging modalities (26). However, a substantial learning curve exists with laparoscopy and thoracoscopy. In addition, invasive staging usually requires 3 to 4 hours of general anesthesia and 2 to 3 days of hospitalization.
Esophageal Carcinoma Staging: TNM
Tumor
The T stage of esophageal carcinoma has marked prognostic implications (5-year survival is approximately 80% for patients with Tis or T1 disease, approximately 65% for those with T2 tumors, 25% for those with T3 disease, and nonexistent with T4 tumors) (27). Furthermore, the T stage correlates with the chance of nodal involvement (0% with Tis, 10% with T1, 33%–50% with T2, 70%–80% with T3, and 85%–90% with T4 tumors) (28,29,30,31,32). Although the optimal treatment of different stages of esophageal cancer lacks consensus, most institutions base treatment protocols on the clinical T and N staging. Therefore, determining the ability of a clinical staging test to correctly predict the pathological T status is important, particularly with regard to the differentiation between Tis through T2, T3, and T4 tumors.
Historically, accurate T staging of esophageal cancer occurred in only 25% to 30% of cases using barium studies, endoscopy, and early generation CT scans (33). Current generation helical CT scanners have much better resolution, and various indices of reliability for these tests are shown in Table 17.3. In the studies shown in Table 17.3, the gold standard was pathological staging at the time of surgical exploration. The criteria for tumor wall invasion by CT and EUS as defined in Table 17.1 are well accepted and used consistently in these studies. Table 17.3 and subsequent tables display data from relevant studies involving 25 or more patients and report data from which FN and FP rates could be calculated. Although the FN rates from studies with high prevalence rates (>90%) and the FP rates from studies with low prevalence rates (<10%) are shown, they are excluded from calculated averages because of questionable validity.
The FP rates for CT in predicting T3 and T4 involvement are consistently low (7%), whereas the FN rate is high (57%). Thus, a CT suggesting T3 or T4 disease is reliable, but a CT suggesting tumor confined to the muscularis propria is not reliable. One-half to two-thirds of advanced tumors in these studies involved T3 and not T4 tumors. Although the presence of a T3 tumor may alter some physicians’ recommendations for therapy, in the opinion of most physicians, the presence of a T4 tumor is a much clearer contraindication to surgical resection. Tumors with T4 involvement have not been consistently addressed as a group, but both aortic and tracheal invasion (the major categories of T4 involvement) have been addressed individually. The results in Table 17.3 indicate that the FN rate of CT is low in these instances, but the FP rate is high. This means that one should be very cautious in excluding patients from surgery on the basis of a CT scan that suggests aortic or tracheal involvement. Conclusions drawn from the reported data are fairly convincing in general, even though there is a fair amount of unexplained variability in the FP rates among individual studies. MRI has demonstrated similar sensitivity, specificity, and FP and FN rates for the determination of T status (4,7).
EUS is largely accepted as the gold standard, nonsurgical, staging modality for accurately determining T status in esophageal cancers (Table 17.3). The ability to predict T3 or T4 tumors from earlier stage T1 and T2 tumors is quite good, with low FP and FP rates (average, 5% and 7%, respectively). In 20% to 30% of cases, the probe cannot be passed through the tumor, given that EUS probes are typically >12 mm in size (30,31,34,35). Hordijk et al. showed that the traversability of lesions also impacts T staging, finding that accuracy decreased from 92% to 46% if lesions were difficult to pass as compared to easily traversable lesions (36). Attempts at dilating nontraversable lesions are fraught with unacceptably high perforation rates, nearly one-fourth of patients in one early series (37). Attempts at staging tumors proximal to the stricture have shown very high FN rates (35), whereas attempts at dilation have had equally poor results (38). Despite these potential drawbacks, EUS is currently the single best staging modality for determining T stage in patients with esophageal cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree