Epithelial Tumors of the Small Intestine
General Features of Small Intestinal Tumors
Benign and malignant small intestinal tumors are uncommon. Most small bowel malignancies are metastases from tumors arising elsewhere (1). Primary small bowel tumors constitute only 1% to 3% of all primary gastrointestinal (GI) malignancies (1,2,3,4) and <2% of all human malignancies (1). The major malignant tumors that arise in this location include adenocarcinomas, lymphomas (discussed in Chapter 18), carcinoid tumors (discussed in Chapter 17), and GI stromal tumors (discussed in Chapter 19). Data from the Surveillance, Epidemiology, and End Results (SEER) Program found that small bowel tumors had an annual average incidence rate of 9.9 per million people (5). Carcinoid tumors and adenocarcinomas were the most common histologic types with an average annual incidence rate of 3.8 and 3.7 per million people, respectively, followed by stromal tumors and lymphomas (5). The small intestine also gives rise to a number of benign lesions including Brunner gland lesions, adenomas, and a variety of polyps that are often a component of a polyposis syndrome (see Chapter 12). Table 7.1 shows the World Health Organization (WHO) classification of small intestinal tumors (6).
Proliferative Brunner Gland Lesions
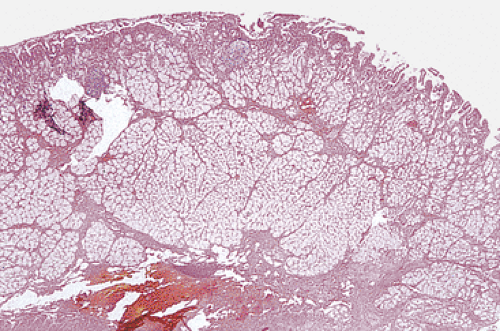
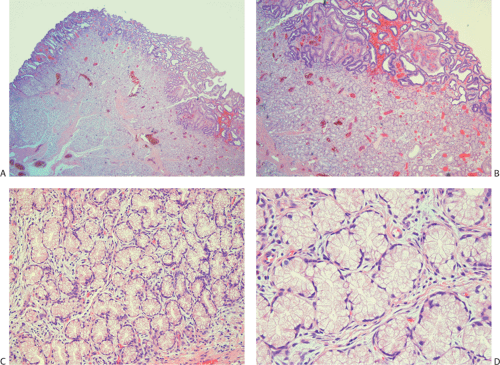
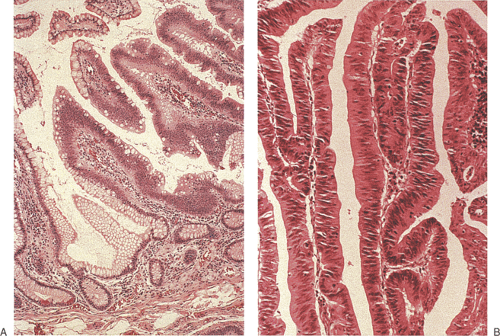
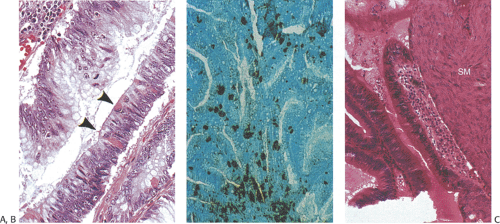
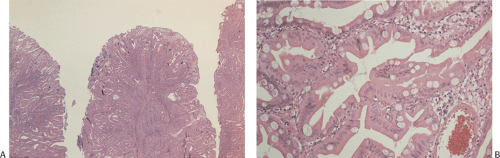
Enlarged Brunner gland lesions present endoscopically as a submucosal mass. The enlargement most commonly results from hyperplasia, although rare neoplastic lesions also develop (Fig. 7.1). Brunner gland hamartomas and true Brunner gland adenomas do exist, but adenomas are much less common than reported in the literature. The lesions generally affect older individuals and arise on the posterior wall of the duodenum. They are usually detected at the time of upper endoscopy, although they occasionally become symptomatic causing vomiting, bleeding, or obstruction. Both hamartomas and adenomas usually retain their lobular architecture (Fig. 7.1). Hamartomas have fibrous septa coursing between the hyperplastic lobules. They may be accompanied by ciliated cysts and prominent adipose tissue (7). Prominent ducts can be seen as well. The diagnosis of Brunner gland adenomas is based on both architectural and cytologic features. There may be mild architectural distortion and the glands appear more crowded than usual (Fig. 7.2). Cytologically, the nuclei are enlarged and they may be overlapping. These neoplastic cells merge imperceptibly with more normal-appearing epithelial cells. Mitotic figures are rare. This lesion may associate with peptic duodenitis (Fig. 7.2). Rarely, Brunner gland adenomas develop atypical hyperplasia (Fig. 7.2) or undergo malignant transformation (8,9).
Nonneoplastic Intestinal Epithelial Polyps
A number of nonneoplastic polyps develop in the small intestine, including Peutz-Jeghers polyps and juvenile polyps. These are often part of a polyposis syndrome and occasionally these polyps contain areas of malignancy. They are discussed in Chapter 12. Lymphoid polyps can also develop in the small intestine and they are discussed in Chapter 18.
Intestinal Adenomas
Small intestinal adenomas are rare, constituting <0.05% of all intestinal adenomas (10). Since most adenomas arise near the ampulla of Vater, it is postulated that carcinogens or cocarcinogens present in bile or pancreatic secretions play a role in their development. Bile salts are believed to act as tumor promotors, especially when combined with acid from the stomach (11). However, no specific dietary substance, chemical, or toxin is a demonstrated etiologic agent responsible for adenoma production. The only clear risk factor for developing small intestinal adenomas is the presence of one of the genetically inherited polyposis syndromes (see Chapter 12) or the presence of an underlying condition (Table 7.2). A rare example of fraternal sisters with adult polycystic kidney disease and ampullary adenomas also raises the possibility of a genetic link between autosomal dominant polycystic kidney disease and ampullary adenomas (12).
TABLE 7.1 World Health Organization Classification of Small Intestinal Tumors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Patients with small intestinal adenomas range in age from 30 to 90 years, with a peak incidence in the 7th decade. Patients with adenomas are generally younger than those with carcinomas or adenomas containing carcinoma (13). Adenomas affect both sexes equally. Many small adenomas remain asymptomatic, only to be discovered incidentally at the time of upper endoscopy for other reasons. They may also be found in those undergoing endoscopic surveillance because they have a polyposis syndrome or have a family history of a polyposis syndrome. Large lesions become
symptomatic. Ampullary adenomas present as biliary colic, biliary obstruction, cholangitis, jaundice, pancreatitis, and/or pain (10). Partial or total intestinal obstruction, low-grade bleeding, cramps, vomiting, nausea, anorexia, weight loss, intussusception, or hemorrhage may also develop depending on adenoma size and location. Villous adenomas tend to be larger and are more likely to become symptomatic than smaller tubular adenomas. Rare patients with secretory villous adenomas present with mucorrhea and electrolyte imbalances (14).
symptomatic. Ampullary adenomas present as biliary colic, biliary obstruction, cholangitis, jaundice, pancreatitis, and/or pain (10). Partial or total intestinal obstruction, low-grade bleeding, cramps, vomiting, nausea, anorexia, weight loss, intussusception, or hemorrhage may also develop depending on adenoma size and location. Villous adenomas tend to be larger and are more likely to become symptomatic than smaller tubular adenomas. Rare patients with secretory villous adenomas present with mucorrhea and electrolyte imbalances (14).
TABLE 7.2 Conditions That Predispose Patients to Develop Small Intestinal Epithelial Neoplasms | |
|---|---|
|
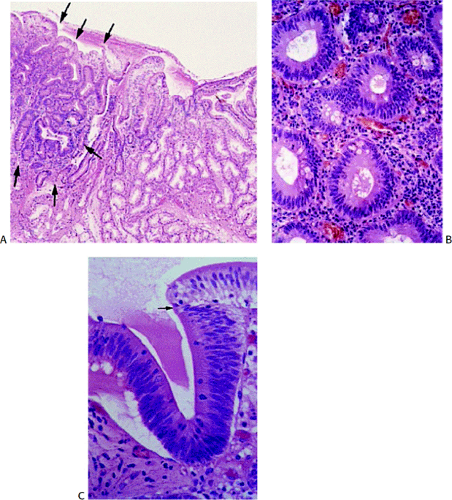
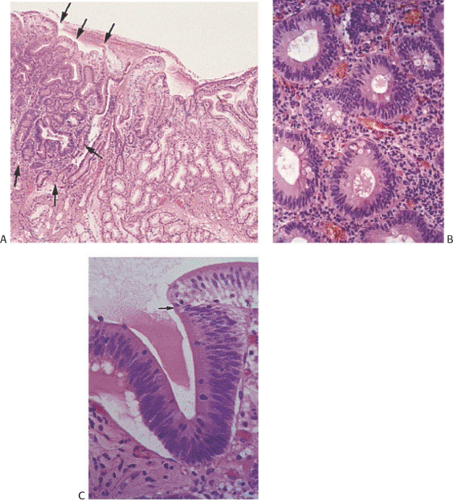
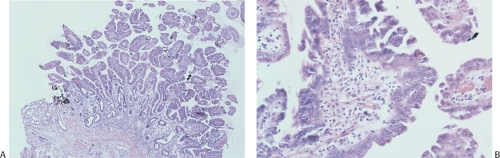
Adenomas appear as soft, lobulated, pedunculated or sessile, single or multiple lesions (Fig. 7.3). The mucosa may have a discolored granular appearance, but without erosions or ulcers (15). Evenness of the granularity helps distinguish adenomas from cancers. Sessile adenomas are more frequent than pedunculated ones. Tubular adenomas vary in size from 0.5 to 3 cm in maximum diameter. Villous adenomas are usually larger and may attain a size of ≥8 cm. Large villous adenomas may encircle the bowel lumen (1) and appear as a cauliflowerlike lobulated sessile polypoid mass. Villous adenomas may also be encountered in the jejunum. The presence of multiple small intestinal adenomas suggests that the patient has a polyposis syndrome (see Chapter 12) or an underlying disorder such as Crohn disease (16).
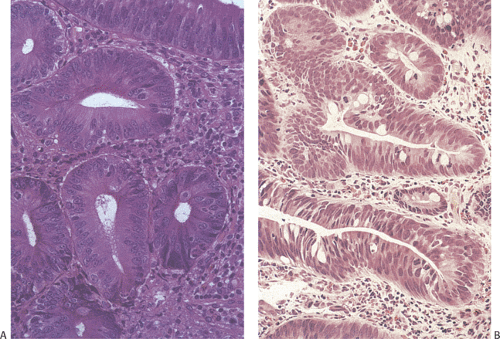
Adenomas are benign neoplasms that display varying degrees of dysplasia. They are tubular (Fig. 7.4), tubulovillous, or villous (Fig. 7.5) in nature, resembling their colonic counterparts. Tall, immature columnar, pseudostratified epithelial cells displaying a typical “picket fence” pattern line the neoplastic tubules (Figs. 7.5, 7.6, 7.7, and 7.8). Goblet cells that exhibit variable degrees of differentiation lie among the immature enterocytes. Sometimes the goblet cells appear to be dystrophic as evidenced by the presence of signet ring cells. Small intestinal adenomas can also contain endocrine cells (Fig. 7.6), Paneth cells (Fig. 7.7), and squamous cells
(Fig. 7.7), attesting to their origin from multipotential crypt stem cells. Paneth cells may be quite numerous, especially in patients with familial polyposis. Variable degrees of nuclear atypia affect all cell types present within adenomas, supporting the concept that each represents an inherent neoplastic component of the lesion and not entrapped normal cells. In small lesions, the adenomatous epithelium may be confined to the surface and not involve the crypts. Normal lamina propria separates the neoplastic glands (Figs. 7.4, 7.5, 7.6, 7.7, and 7.8).
(Fig. 7.7), attesting to their origin from multipotential crypt stem cells. Paneth cells may be quite numerous, especially in patients with familial polyposis. Variable degrees of nuclear atypia affect all cell types present within adenomas, supporting the concept that each represents an inherent neoplastic component of the lesion and not entrapped normal cells. In small lesions, the adenomatous epithelium may be confined to the surface and not involve the crypts. Normal lamina propria separates the neoplastic glands (Figs. 7.4, 7.5, 7.6, 7.7, and 7.8).
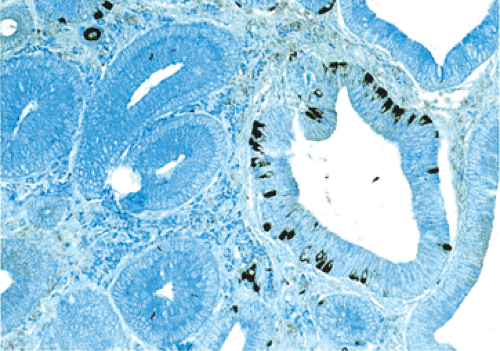 FIG. 7.6. Chromogranin immunostaining of small intestinal adenoma demonstrating the presence of numerous neuroendocrine cells (dark brown cells). |
A small villous component may be present on the surface, but most of the lesion should consist of tubules to classify it as a tubular adenoma (Fig. 7.4). Villous adenomas consist of fingerlike villous or papillary processes containing central thin cores of lamina propria lined by a neoplastic epithelium resembling that seen in tubular adenomas (Fig. 7.5). Occasionally one sees mixed tubulovillous adenomas.
A full spectrum of neoplasia can be seen in small intestinal adenomas, ranging from low-grade dysplasia to high-grade dysplasia to invasive carcinoma. The probability of finding areas of carcinoma depends on the size and location of the lesion (17). The larger the tumor, the more likely one is to find invasive cancer and the less likely one is to see residual adenoma. As the dysplasia becomes more severe, the nuclear:cytoplasmic ratio increases, epithelial polarity disappears, and the cells demonstrate increased mitotic activity (Figs. 7.8 and 7.9). The nuclei consistently approach the glandular lumens in high-grade dysplasia. Marked glandular budding with loss of nuclear polarization and variable loss of mucinous differentiation heralds the development of malignancy. If one sees severe dysplasia with unequivocal invasion into the lamina propria, with a back-to-back glandular
configuration and glandular fusion, one can make the diagnosis of intramucosal carcinoma.
configuration and glandular fusion, one can make the diagnosis of intramucosal carcinoma.
Serrated Adenomas
We have encountered occasional examples of serrated duodenal adenomas and there is one reported case of this lesion (18). They resemble their large intestinal counterparts. These adenomas have serrated lumens lined by eosinophilic-appearing cells that contain pseudostratified nuclei with prominent nucleoli (Fig. 7.10). Goblet cells are typically not well developed in these lesions. These lesions are too uncommon in the duodenum to comment on the implications of this histologic variant. However, we have not seen any containing either high-grade dysplasia or carcinoma.
Interpretation of Biopsies with Areas Suspicious for Epithelial Neoplasia
Typically, the initial diagnosis of a duodenal neoplasm involves interpretation of small biopsy specimens that yield only a small sample of the superficial parts of the lesion. The deeper parts of an adenoma, where an invasive tumor is most likely to develop, are often not present in the biopsy. For this reason, it is difficult to exclude the presence of an invasive cancer, especially in larger lesions. In one study of duodenal villous adenomas, biopsies missed areas of malignancy in 56% of cases, indicating the poor sensitivity of biopsies in detecting an invasive cancer (19). Generally, the best that one can do is to recognize that the lesion is neoplastic and to provide an accurate assessment of the degree of dysplasia that is present, stating whether there is invasion into the lamina
propria and whether one sees lymphovascular invasion or desmoplasia. One can also state whether submucosal tissue is present to be evaluated for the presence of invasion. If severe dysplasia or intramucosal carcinoma is present, consideration should be given to resection of the lesion, because it may harbor an invasive malignancy. The absence of identifiable lamina propria surrounding glands, the presence of large vessels near the tumor cells, a desmoplastic response, and an intravascular or intralymphatic invasion all support a diagnosis of invasive cancer. Tumors with high-grade dysplasia or a villous morphology are more likely to harbor an invasive carcinoma than adenomas lacking these features (19). Larger, ulcerated lesions that are fixed or cause obstruction usually contain invasive cancer.
propria and whether one sees lymphovascular invasion or desmoplasia. One can also state whether submucosal tissue is present to be evaluated for the presence of invasion. If severe dysplasia or intramucosal carcinoma is present, consideration should be given to resection of the lesion, because it may harbor an invasive malignancy. The absence of identifiable lamina propria surrounding glands, the presence of large vessels near the tumor cells, a desmoplastic response, and an intravascular or intralymphatic invasion all support a diagnosis of invasive cancer. Tumors with high-grade dysplasia or a villous morphology are more likely to harbor an invasive carcinoma than adenomas lacking these features (19). Larger, ulcerated lesions that are fixed or cause obstruction usually contain invasive cancer.
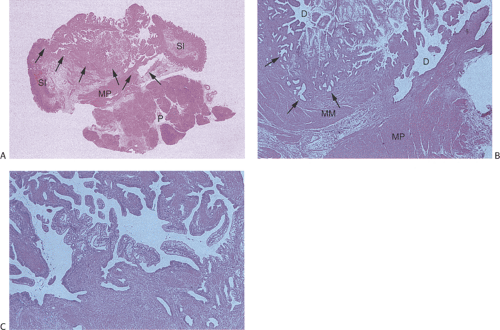
Special caution must be applied to the evaluation of ampullary lesions because the anatomy of this area is quite complex and numerous small branched submucosal glands normally reside in this region (see Chapter 6). A significant diagnostic dilemma results when carcinoma in situ or intramucosal cancer involves these submucosal glands (Figs. 7.11 and 7.12). It is easy to confuse involvement of ampullary glands with invasive disease, and care needs to be taken not to overdiagnose invasive malignancy in this setting. If one sees a lobular glandular architecture with lamina propria surrounding the glands, invasive malignancy is unlikely. Further, if the glands are round and not angulated, the lesion is more likely to be benign. The lack of desmoplasia also favors a benign lesion.
It is important not to mistake regenerative atypia present on the eroded surface of an adenoma for high-grade dysplasia or an invasive cancer. Areas of acute inflammation containing prominent capillaries and fibrin deposits, especially when superficial, should alert the examiner to the possibility of reparative atypia in the setting of surface erosion.
Another common area of diagnostic error is the presence of the marked reactive atypia that can be present in the duodenum, especially in the area surrounding the ampulla of Vater in patients with sclerosing papillitis (see Chapter 6). Patients who have had stents or who have had cholelithiasis may exhibit very significant ampullary inflammation, often accompanied by a marked papillary hyperplasia with significant reactive atypia. The papillary hyperplasia can appear polypoid and may even obstruct the ampulla—leading to secondary alterations in the biliary tree or pancreatic ducts. These circumstances lead to the strong clinical impression that the patient has a neoplasm. We see many cases of reactive atypia that were misdiagnosed as adenoma, often as adenoma with high-grade dysplasia. One should exercise caution in making a diagnosis of dysplasia in the presence of acute inflammation. We find that Ki-67 immunostaining can be helpful in distinguishing between a reactive and a neoplastic lesion. In reactive lesions, one usually sees a proliferative
zone that extends upward from the base of the crypt. Not all cells exhibit positive staining. In contrast, neoplastic lesions often have much stronger Ki-67 staining that is localized superficially and not in the crypt base, especially if the lesion is early. If one is unable to distinguish a reactive from a neoplastic process, a diagnosis of indefinite for dysplasia is appropriate.
zone that extends upward from the base of the crypt. Not all cells exhibit positive staining. In contrast, neoplastic lesions often have much stronger Ki-67 staining that is localized superficially and not in the crypt base, especially if the lesion is early. If one is unable to distinguish a reactive from a neoplastic process, a diagnosis of indefinite for dysplasia is appropriate.
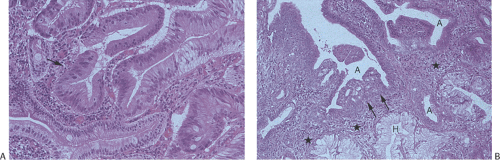 FIG. 7.12. Higher magnification of the lesion illustrated in Figure 7.11. A: Stratified epithelium lines the glandular spaces. The glands are separated by normal-appearing lamina propria. In some foci the epithelium appears more dysplastic than in others (arrow). These same glands also lack normal mucinous differentiation. B: This area of the tumor demonstrates areas of glandular hyperplasia (H) as well as foci of adenoma (A). One of the adenomatous glands contains an area of intramucosal carcinoma (arrow). Normal lamina propria separates the hyperplastic and adenomatous glands (stars). Note the absence of desmoplastic stroma. This neoplasm is of the hepatobiliary type. |
Mixed Hyperplastic and Adenomatous Polyps of Gastric Origin
Rare polypoid lesions that contain both hyperplastic and adenomatous gastric mucosa arise in areas of heterotopic gastric mucosa. The hyperplastic foci resemble those seen in gastric hyperplastic polyps. These hyperplastic foci intermingle with adenomatous epithelium indistinguishable from small intestinal adenomas (20). The lesions are histologically identical to similar lesions arising in the stomach (see Chapter 4).
Carcinomas
Epidemiology
Relative to the length and surface area of the small intestine, adenocarcinomas are quite rare. It was estimated that in 2006 there were approximately 6,000 new cases of small intestinal cancer in the United States (21). Most carcinomas develop in the duodenum and their incidence is increasing slightly due to the increased use of upper endoscopy and enteroscopy. As a result, they are commonly detected early. Small intestinal carcinomas generally present between the ages of 50 and 70 unless the patient has an underlying inflammatory condition or polyposis syndrome. The median age at presentation is approximately 55 to 67 years. However, these tumors have been described in children as young as 12 (22). The frequency of small bowel cancer roughly parallels the frequency of colon cancer among the participating registries of the International Association of Cancer Registries (23). The U.S. registries for the years 1993–1997 showed the highest rates, followed by Canada and Western Europe, with the lowest rates in Africa and East Asia. Ethnic variations in small bowel cancer in the United States constitute an interesting example of this trend. African-American small bowel cancer rates (male, 2.4; female, 1.8) are double those of U.S. Caucasians (male, 1.2; female, 0.9) and, although the differences are not as large, their large bowel rates are also higher than those of U.S. Caucasians for both sexes. A population-based study of 13 of these registries found 10,946 small bowel cancers among over 4 million first primary cancers (24). There were 4,096 small bowel carcinomas (37.4%), compared to 3,991 carcinoids (36.5%), 1,334 sarcomas (12.2%), 442 lymphomas (4%), and 1,083 unspecified (9.9%). There was a statistically significant increase in the risk of acquiring a second primary cancer. Second primary cancers of the colorectum and the hepatobiliary tree showed the strongest associations between small bowel cancer (p = 0.01), followed by pancreas and ovary (p = 0.02) and soft part sarcoma (p = 0.06). There were similar associations when small bowel cancer followed cancer at another primary site. When assessed by subsite, cancers proximal to the sigmoid colon showed the strongest associations with small bowel cancer. These associations have been attributed to common genetic defects in mismatch or other DNA repair pathways and to common environmental exposures.
Risk factors for small bowel cancer include dietary factors similar to those implicated in large bowel cancer, smoking (25), alcohol intake, and other medical conditions (Table 7.2) (26,27,28). Adenocarcinomas are four times more common in smokers than in nonsmokers (25). Small intestinal adenocarcinomas develop as sporadic lesions or they arise on a background of chronic inflammation (Crohn disease or celiac disease) or in the setting of a polyposis syndrome. Patients with familial adenomatous polyposis and its variant syndromes have a greatly increased incidence of small intestinal adenomas and carcinomas. In fact, upper GI cancer, especially involving the periampullary region, represents a major cause of death in these patients (29). Patients with hereditary nonpolyposis colon cancer (HNPCC) and germline mutations of hMSH2 or hMLH1 have an approximately 4% lifetime risk of small intestinal cancer, which exceeds that in the normal population 100-fold (30). The tumors tend to develop at a younger age than in other polyposis patients, often arising in the jejunum or ileum as well as in the periampullary region.
The risk of developing carcinoma in the setting of Crohn disease is increased up to 18-fold that of the general population (31). The risk of tumor development in Crohn disease may be increased by treatment with 6-mercaptopurine (32). The average age at diagnosis is 48 years and there is a male preponderance. The tumors tend to arise primarily in the ileum but also are seen in the jejunum. The risk of tumor development relates to the disease duration. The tumors are often multifocal and appear to progress through a dysplasia–carcinoma sequence.
Celiac disease also increases the incidence of small bowel adenocarcinoma but adenocarcinomas are not as frequent as enteropathy-associated lymphomas. Celiac disease–associated carcinomas account for approximately 10% of all small intestinal carcinomas (33). These adenocarcinomas predominantly affect the duodenum and upper jejunum, although occasionally they arise in the distal jejunum (34). Multiple synchronous or metachronous tumors may develop in this setting (35).
Adenocarcinomas also arise in ileostomies and ileal conduits, with the time interval between the formation of the ileostomy and carcinoma development usually involving several decades (36). Many develop in patients with inflammatory bowel disease (IBD) who had either antecedent backwash ileitis or dysplasia (36). Ileal pouches fashioned as part of the treatment of adenomatous polyposis, or ulcerative colitis, may develop adenocarcinomas, although the risk is low (37). Ileal carcinomas may also develop in the setting of abetalipoproteinemia (38). Finally, ampullary carcinomas may affect siblings without a known polyposis syndrome, suggesting a possible underlying genetic disorder (39).
Pathogenesis
Adenocarcinomas develop far less frequently in the small intestine than they do in the colon or rectum, despite the fact that the small bowel has a high cellular turnover rate and one of the largest epithelial surfaces in the body. More than 50% to 70% of small intestinal carcinomas arise in the duodenum in the periampullary region, even though it constitutes only 4% of the entire small intestine. The longest small intestinal segment, the ileum, is most resistant to tumor formation unless the patient has Crohn disease.
The preponderance of duodenal carcinomas at the ampulla of Vater may suggest that some component(s) of the biliary or pancreatic secretions play a role in their genesis. Alternatively, the constant influx of alkaline bile and/or acidic pancreatic juice may cause local cell damage. Increased mitotic activity associated with mucosal repair from injury induced by these secretions may predispose to tumor development (40).
The reasons for the relative rarity of small bowel carcinomas in contrast to colon carcinomas remain unknown, but several explanations exist (41,42,43,44).
The luminal contents are more liquid in the small intestine than in the large intestine and potential luminal carcinogens become diluted, leading to reduced mucosal contact.
Small intestinal transit time is rapid as compared to colonic transit time so that potential intraluminal carcinogens have shorter mucosal contact times.
The small bowel usually lacks the anaerobic bacteria present in the large bowel that are capable of converting bile salts to potential carcinogens (42). (When bacterial flora is disturbed, as in bacterial overgrowth syndromes, small intestinal carcinomas develop with a higher frequency than expected.) The antibacterial function of Paneth cells may contribute to the relative sterility of the small bowel lumen (43).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree