A validated disease-specific symptom-assessment tool for eosinophilic esophagitis (EoE) has yet to be approved by regulatory authorities for use in clinical trials. Relevant end points for daily practice include EoE-related symptoms and esophageal eosinophilic inflammation. Endoscopic features should also be taken into account when establishing a therapy plan. A reasonable clinical goal is to achieve a reduction in EoE-related symptoms and esophageal eosinophilic inflammation. Evidence is increasing to support an anti-inflammatory maintenance therapy, as this can reduce esophageal remodeling. In EoE patients in clinical remission, annual disease monitoring with symptom, endoscopic, and histologic assessments of sustained treatment response is recommended.
Key points
- •
Recommended therapeutic end points in eosinophilic esophagitis (EoE) include symptoms (eg, dysphagia, chest pain), histologic activity, and endoscopic activity (especially strictures). Symptom assessment should examine meal modification and food-avoidance behaviors.
- •
Evidence is accumulating that maintenance therapy for EoE, by means of either swallowed topical corticosteroids or elimination diets, leads to a reduction of symptoms and esophageal remodeling processes that are associated with food bolus impactions.
- •
Esophageal dilation can offer long-lasting symptom improvement for EoE patients with esophageal remodeling not responsive to medical or diet therapy.
Introduction
This article covers several clinically relevant topics in the clinical management of eosinophilic esophagitis (EoE). First, which end points should be assessed in daily practice and in clinical trials are discussed. Second, the existing evidence to support maintenance treatment is highlighted, and the different therapeutic options discussed. Third, treatment options for patients refractory to standard therapies and for asymptomatic patients with esophageal eosinophilia are addressed. Finally, a therapeutic algorithm is presented.
Introduction
This article covers several clinically relevant topics in the clinical management of eosinophilic esophagitis (EoE). First, which end points should be assessed in daily practice and in clinical trials are discussed. Second, the existing evidence to support maintenance treatment is highlighted, and the different therapeutic options discussed. Third, treatment options for patients refractory to standard therapies and for asymptomatic patients with esophageal eosinophilia are addressed. Finally, a therapeutic algorithm is presented.
End points to assess treatment efficacy
General Considerations
EoE has been defined as a clinicopathologic entity with symptoms of esophageal dysfunction and eosinophil-predominant esophageal inflammation.
EoE activity can be assessed by patient-reported outcomes (PRO) in addition to biological markers, including endoscopic and histologic alterations as well as serologic biomarkers. One should discriminate between the use of outcomes in daily clinical practice and in clinical trials. For daily clinical practice the relevant outcomes are EoE-related symptoms, esophageal eosinophilia, and endoscopic features, especially the presence of esophageal strictures. In addition to these outcomes, quality of life and different biomarkers can become relevant in clinical trials.
Fig. 1 provides an overview of the different dimensions in which EoE activity can be measured. There is an ongoing debate as to whether EoE activity should be assessed based on PRO, biological components, or both dimensions. Which measures most accurately reflect disease activity depends on the impact of the disease’s natural history on either PRO or biological markers. This concept is further illustrated by Fig. 2 . In diseases such as migraine (PRO = headache; biological marker = abnormalities in functional magnetic resonance imaging [MRI]) or low back pain (PRO = pain; biological marker = MRI findings), activity assessment is mainly based on PRO measurement. At the other end of the spectrum there exist diseases in which patients may remain without any symptom for a long time but whereby appropriate biomarkers exist for activity assessment that are associated with distinct clinical outcomes, such as myocardial infarction in arterial hypertension (PRO = eg, quality of life; biological marker = blood pressure). In between these two poles there are diseases such as inflammatory bowel diseases (PRO = eg, bowel frequency, abdominal pain; biological marker = severity of endoscopically assessed inflammation) in which activity is determined by both PRO and biological markers.
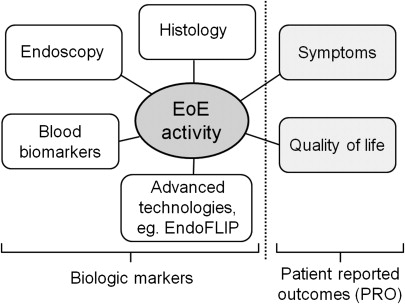
Straumann and colleagues, the first to publish on the natural history of 30 adult EoE patients, demonstrated that symptoms and eosinophil-predominant esophageal inflammation persisted over time. Several recent publications have demonstrated that long-standing eosinophil-predominant esophageal inflammation leads to deposition of subepithelial fibrous tissue, and that this remodeling process is associated with stricturing complications. Current understanding suggests that symptom generation in EoE depends on active, eosinophil-predominant esophageal inflammation and associated esophageal remodeling processes. These observations support the recommendation that EoE activity assessment in daily practice and clinical trials should be performed using a combination of PRO and biological markers.
There is currently no validated activity index to measure EoE activity in the different dimensions. Such an index is urgently needed to define end points for clinical trials, observational studies, and daily clinical practice. Several therapeutic trials have reported either a correlation or a dissociation between EoE-related symptoms and esophageal eosinophil counts, which might be related to the use of different, mostly nonvalidated instruments for symptom assessment. The lack of a standardized, validated PRO instrument to assess EoE-associated symptom severity has several important implications. First, the results of different clinical trials are difficult to compare. Second, several therapeutic trials have documented heterogeneous associations between changes in PRO and biological markers. As such, the current situation poses a challenge for regulatory authorities to approve therapies for EoE management. The US Food and Drug Administration (FDA) has identified the deficiency of clinically meaningful end points in EoE, calling for effective collaboration involving different interest groups (patients, physicians, researchers, pharmaceutical industry, and regulatory authorities).
Current Status Regarding the Development of PRO Instruments
The development and validation of a PRO instrument to assess symptom severity in pediatric and adult EoE patients represents a challenge for several reasons. First, the leading EoE symptoms typically change in the pediatric population with ongoing age. Second, again for pediatric patients, a cutoff age has to be chosen from which children are able to report on symptoms themselves; moreover, up to which age symptom reporting should be performed by parents must be decided. Third, the severity and frequency of dysphagia, which represents the leading symptom in adolescent and adult EoE patients, strongly depends on the ingested food consistencies; therefore, symptoms should be assessed according to defined food categories. Fourth, symptom severity may depend on food avoidance, food modification, or the time to ingest a standardized meal. These behavioral modifications should also be taken into account when developing a PRO instrument. Fifth, a distinct symptom-recall period has to be chosen for symptom assessment. The choice of the optimal symptom-recall period depends, among other factors, on the intended use of the PRO, the patient’s ability to recall the required information, and the extent to which the patient is burdened by his or her EoE when completing the instrument. All these factors should be considered when developing a PRO instrument for pediatric and adult EoE patients. The performance of an esophageal stress test with ingestion of a standardized meal to measure symptom severity bears the potential risk of acute food bolus impaction, and should therefore be exercised with caution.
Several PROs are being evaluated for EoE. The Dysphagia Symptom Questionnaire (DSQ) is a 3-item electronic PRO that was developed for the purpose of a pharmaceutical trial for EoE. The DSQ is administered daily to assess the frequency and intensity of dysphagia caused by eating solid food. The Eosinophilic Esophagitis Activity Index (EEsAI) study group is currently developing and validating a PRO instrument to assess EoE symptom severity. The EEsAI PRO instrument evaluates dysphagia severity according to 8 distinct food consistencies, and also takes into account behavioral adaptations such as food avoidance, food modification, and time to eat a regular meal ( clinicaltrials.gov , NCT00939263 ). In 2011 Taft and colleagues published a quality-of-life questionnaire for adult EoE patients. The Adult EoE Quality of Life (EoO-QOL-A) Questionnaire demonstrated good internal consistency and test-retest reliability. Franciosi and colleagues reported in 2011 on the qualitative methods of the Pediatric Eosinophilic Esophagitis Symptom Score (PEESS, version 2.0). The same group recently published a quality-of-life instrument for pediatric EoE patients.
Overview of the Current Status Regarding the Development of Biological Measures
A classification and grading system for endoscopic assessment of esophageal features in EoE was recently published. The EoE Endoscopic REFerence Score (EREFS) assesses 5 characteristic features of exudates, rings, edema (loss of vascular markings), furrows (longitudinal markings), and stricture. Definitions for endoscopic remission and mild, moderate, or severe endoscopic activity still need to be established.
The assessment of histologic activity is mainly based on the peak eosinophil count per high-power field (hpf) or the eosinophil load. Additional histologic findings such as papillary elongation, basal layer hyperplasia, eosinophil degranulation, or subepithelial fibrosis have also been reported as parameters of histologic outcome. Dellon and colleagues have shown that the number of reported peak eosinophil counts may not be necessarily comparable, as different microscope types with specific hpf sizes are being used. One way to overcome this issue would be the reporting of peak eosinophil counts standardized to mm 2 . In analogy to the reporting of endoscopic severity, definitions regarding histologic remission and different semiquantitative degrees of histologic activity still need to be established.
Furuta and colleagues have recently evaluated the esophageal string test as a minimally invasive tool to assess the correlation between esophageal eosinophil counts and eosinophil granule proteins attached to the string. Excellent correlations were found between the level of eosinophil granule proteins extracted from the string and esophageal tissue eosinophilia. Long-standing eosinophil-predominant inflammation leads to esophageal remodeling, resulting in stricture formation. The EndoFLIP, an impedance planimetry catheter–based device, measures esophageal compliance and distensibility and is thereby able to provide quantitative information on remodeling consequences in EoE.
Maintenance therapy
Only recently have studies begun to address the need and management options for long-term maintenance therapy in EoE. In examining the topic of maintenance therapy, limitations to existing assumptions should be acknowledged. (1) Although EoE is considered a chronic and lifelong disease, only 10 to 20 years of data exist, and spontaneous remission could occur. (2) The concept that untreated disease leads to a progressive fibrosis and stricture formation is largely based on retrospective data. (3) The current focus on control of esophageal eosinophilia as the end point of therapy ignores other potentially significant inflammatory pathways (eg, eosinophil degranulation proteins) and cells (eg, mast cells, basophils, lymphocytes).
Why is Maintenance Needed?
As in any chronic disease, the decision to use maintenance therapy balances the benefits of symptom control and prevention of disease progression with costs, side effects, and complications of long-term therapy. In the case of EoE, those in favor of observation and periodic treatment of symptom exacerbations might point out that many patients with EoE adapt to their disease, weight loss is uncommon, the disease remains isolated to the esophagus, and there are no neoplastic consequences.
On the other hand, EoE may be associated with morbidity. For example, food impaction is common, occurring in up to 35% of patients. Patients with food impaction are indubitably at risk for perforation and aspiration. Furthermore, spontaneous perforation during food impaction (Boerhaave syndrome) has been reported. Perforation may also occur with endoscopic bolus disimpaction and esophageal dilation. Although uncommonly requiring surgical repair, esophageal perforation results in chest pain, and careful inpatient observation is necessary. The impact of EoE on the quality of life is now being examined. Patients are commonly anxious or embarrassed by their slow eating and/or diet restrictions. As a result, important events such as business meals or social gatherings may be avoided. This scenario is particularly problematic among teenagers and young adults, a common demographic of the disease, of an age at which social stigmatisms are easily perceived.
The reason such complications ensue is the high rate of stricture formation. Indeed, once patients with EoE become or are diagnosed as young adults, the rate of esophageal stenosis reaches up to 40%. In untreated patients, the natural history of persistent esophageal eosinophilia and increased collagen deposition supports the risk of progression. In patients with initial successful treatment, disease regression is uncommon. In a recent study from the Swiss EoE database, the duration of untreated disease (as measured through years of untreated symptoms) corresponded to the chance of stricture formation. Specifically, after 30 years of untreated symptoms of EoE, 80% of patients had esophageal stricture formation. Of note, these strictures were diagnosed with endoscopy. If one uses more sensitive tests to diagnose esophageal fibrosis, such as barium esophagography, endoscopic ultrasonography, or the EndoFLIP, the prevalence of strictures is even higher, including patients with a normal-appearing esophagus or alterations limited only to rings. Studies with the EndoFLIP, a measurement of esophageal distensibility whereby patients may demonstrate a marked decrease in esophageal compliance presumably long before endoscopic strictures are evident, are particularly enlightening.
There is also a good foundation of basic research that accounts for esophageal stricture formation in EoE. Specifically, careful analysis of tissue and inflammatory mediators in EoE has demonstrated the profibrotic process that results from the eosinophil-mediated inflammation in EoE. For example, subepithelial collagen deposition is a common finding in these patients. Moreover, many of the inflammatory mediators released from eosinophils are profibrotic. As a result, one of the main justifications for the use of maintenance therapy is to control esophageal inflammation and thereby prevent stricture formation.
Disease chronicity is a strong argument in favor of maintenance therapy. Prospective data demonstrate disease relapse in most, if not all patients, following cessation on initial therapy. However, there are only limited data demonstrating that maintenance therapy prevents or reverses existing esophageal strictures.
What is the Goal of Maintenance Therapy?
The goal of maintenance therapy may focus on symptoms, control of histologic inflammation, or both. For symptom relief, periodic esophageal dilation may be as effective as swallowed fluticasone in reducing dysphagia. Esophageal inflammation, however, is not lessened with dilation alone. Thus, from a pathophysiologic point of view, the goal of maintenance medical therapy is to reduce esophageal eosinophilia and adverse consequences of esophageal remodeling. Unfortunately, as yet there are no data that can guide to what degree esophageal eosinophilia must be reduced, or even whether the eosinophil is the primary determinant of fibrotic change. More specifically, it is not known whether complete elimination of esophageal eosinophils is necessary or if fewer than 5, fewer than 10, or fewer than 15 eosinophils per hpf is sufficient. Indeed, in other chronic inflammatory diseases that lead to fibrosis, such as inflammatory bowel disease, investigators have long debated the appropriate end point of medical therapy, with more recent data suggesting that endoscopic demonstration of mucosal healing predicts sustained control of disease. EoE is now approached in a similar manner, with the desired goal of sustained and complete elimination of eosinophilic inflammation.
Which Patients with EoE Should Be Considered for Maintenance Therapy?
Should all patients with EoE be treated with maintenance therapy? To some degree this depends on the safety and tolerability of the therapy. A patient whose esophageal inflammation remains under control with avoidance of a limited number of foods might continue with diet therapy indefinitely. On the other hand, for patients who use topical steroids, in whom the risk of long-term side effects is unclear, a more selective strategy may be appropriate. As a result, it may be important to identify subsets of EoE patients who are at greater risk of developing esophageal strictures or in whom clinically significant strictures already exist. Such patients might include those with repeated food impactions, those who relapse quickly with symptoms and/or esophageal eosinophilia off therapy, patients who cannot maintain their weight owing to severe diet restrictions, or those with narrow or small-caliber esophagus. It must be borne in mind that given the lack of data in this area, it is not known whether all or any of these specific subsets of EoE patients will respond to therapy. Patients with narrow-caliber esophagus or multiple severe atopic comorbidities may be less responsive to conventional therapies.
What Potential Maintenance Treatments Exist and Are They Effective?
The potential treatments to maintain remission in EoE include steroids and elimination diet therapy. There are several reasons for which the use of steroids for maintenance therapy is an attractive option. First, there are clear data demonstrating excellent control of inflammatory change and reduction of tissue eosinophilia. Second, studies in EoE have shown downregulation of genes associated with tissue remodeling following steroid therapy. Furthermore, there are some clinical data suggesting an increase in stricture diameter with steroids based on endoscopic and/or radiologic assessment. There are additional data demonstrating that steroids are effective in suppressing some of the key pathways and genes that mediate esophageal injury in EoE and the abnormal transcriptome of EoE, thus holding a mechanistic potential to reverse fibrosis. In favor of diet therapy is the lack of concern for side effects as long as daily nutritional requirements are met. On the other hand, diet therapy poses greater challenges with identification of triggering food antigens and in lifelong avoidance of these antigens, particularly when multiple and/or common table foods are implicated. This latter point is particularly relevant given the commonality of milk and wheat allergy identified in these patients. Elemental diets, though also effective, are limited by tolerability and expense.
Another emerging issue in maintenance therapy is whether control of extraesophageal allergies positively affects esophageal disease. This contention is suggested in animal models by induction of esophageal eosinophilia through an initial priming mechanism of allergy in the lung or skin. It is supported in humans by the finding of years of preceding airway allergies in most patients as well as the inconsistent finding of flares of EoE during respiratory allergy seasons. On identification of risk factors for EoE in patients with asthma, patients most typically have an allergic phenotype such as presence of allergic asthma and peripheral eosinophilia. However, no controlled trials have been performed that demonstrate a beneficial effect of therapy directed toward extraesophageal allergic disease on EoE.
Data on Maintenance Therapy
Despite the theoretical benefits of maintenance steroid therapy, there is a distinct paucity of data available. In a randomized controlled trial conducted by Straumann and colleagues, 28 patients were randomized to 0.25 mg of budesonide twice daily or placebo for 50 weeks. The favorable results from this trial demonstrated that budesonide reduced markers of inflammation, epithelial cell apoptosis, and remodeling events, without adverse side effects. Unfortunately, eosinophil count and symptoms significantly increased on both placebo and budesonide. A higher maintenance dosing of budesonide may have improved the therapeutic gain. These findings provide valuable data for defining an adequate maintenance dose, but further work needs to be performed to refine this approach.
Another looming issue on maintenance therapy is the potential long-term side effects of oral steroid therapies. In the Straumann trial, measurement of the effects of long-term budesonide on adrenal function are not discussed. In a 3-month trial examining the use of swallowed topical steroids in children with EoE, there was no clear evidence of adrenal suppression. Similarly, in another pediatric randomized study comparing prednisone with fluticasone, systemic effects such as Cushingoid features were only identified with systemic steroid administration. When inhaled steroids are used for maintenance therapy in patients with asthma, both fluticasone and budesonide have been shown to slightly increase the risk for adrenal suppression. One has to be careful in extrapolating the effects of steroid therapy in asthma to EoE, as the distal esophagus venous network drains through the portal vein, exposing the drugs to hepatic first-pass metabolism. One also needs to consider that although it is assumed that little small-bowel or esophageal absorption occurs with the oral route of a topical steroid preparation, this is also not well studied.
Diet therapy has more robust and longer-term data on maintenance, albeit uncontrolled. In a series of 562 children studied for up to 14 years and treated mostly with diet, only 11 children remained in remission as defined by absence of esophageal eosinophilia. On the other hand, improvement in symptoms and histology occurred in 98% of 381 patients studied long term and maintained on diet therapy, including 16% maintained on an elemental diet. Lucendo and colleagues reported that adult EoE patients on an empiric 6-food elimination diet had prolonged clinical and histopathologic remission for up to 3 years of follow-up.
Conclusions on Maintenance Therapy
Current data support the conceptual and clinical benefits of long-term maintenance therapy in EoE. At present, however, the appropriate patients to use maintenance therapy, the proper end point of therapy that will prevent and perhaps reverse complications of EoE, and the type of therapy that will provide the greatest benefit-to-risk ratio are unanswered questions. Nevertheless, it is reasonable to discuss the potential benefits of maintenance therapy in all patients with EoE, particularly those who have already evidenced disease complications. For those patients who have responded and are able to adhere to elimination diet therapy, maintenance is relatively straightforward. For those patients who have responded to short-term topical steroids, options include reduced-dose maintenance steroids, intermittent steroids, and clinical observation without therapy. Esophageal dilation, discussed next, may offer a long-term symptom improvement in selected patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





