Swallowed fluticasone and oral viscous budesonide are effective first-line therapies for eosinophilic esophagitis in children. Side effects are minimal without evidence of Cushing syndrome, as seen in treatment with systemic corticosteroids. New studies on alternative delivery systems and different corticosteroids (eg, ciclesonide) are encouraging. As knowledge of corticosteroids in eosinophilic esophagitis expands, newer questions continue to arise concerning dose, delivery, and choice of corticosteroids; long-term adverse effects; and maintenance therapies.
Key points
- •
Topical corticosteroids (CSs) (eg, swallowed fluticasone propionate [FP] and oral viscous budesonide [OVB]) are effective first-line therapies for pediatric eosinophilic esophagitis.
- •
Topical CSs have minimal known side effects when used for treatment of eosinophilic esophagitis.
- •
Systemic CSs have significant adverse effects and are now reserved for urgent situations where topical CSs are not effective or in patients who require rapid improvement in symptoms.
The goals for treatment of eosinophilic esophagitis (EoE) are improvements in symptoms and esophageal eosinophilic inflammation with the ideal endpoint complete resolution of the latter. Once a diagnosis of proton pump inhibitor (PPI)-nonresponsive EoE is confirmed, treatment options include pharmacologic agents and/or dietary elimination. If pharmacologic therapy is chosen, topical CSs are effective and considered first line. Although these medications are currently not Food and Drug Administration approved for EoE, the 2 commonly used options are swallowed aerosolized FP and OVB. Systemic CSs (ie, prednisolone and methylprednisolone) may be useful if topical steroids are not effective or in patients who require rapid improvement in symptoms.
This article discusses the use of topical and systemic CSs for induction of remission and as maintenance treatment of pediatric EoE. The risks and benefits of these agents are outlined and some important and clinically relevant questions discussed.
Topical corticosteroids for induction
In 1998, Faubion and colleagues described 4 children with eosinophilic inflammation isolated to the esophagus who improved clinically and histologically by swallowing aerosolized CSs (FP and beclomethasone) from an inhaler without use of a spacer. Over time, FP has become the topical CS used most often in EoE, although other agents are also used (discussed later). We will review prospective and randomized studies involving topical steroids used in pediatric eosinophilic esophagitis ( Table 1 ). Adult studies are discussed elsewhere in this issue.
| Study | Type of Study | Control Group (n) | Histologic Criteria | Drug (n) | Dose (μg) | Length of Treatment | Primary Outcomes | Drug Efficacy a (%) | Control Group Response (%) | Other Outcomes | Adverse Events | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teitelbaum et al, 2002 | Prospective | NA | >15 eos/hpf, superficial layering, and/or eosinophil microabscesses | FP (13) | 2–4 yo: 88 BID 5–10 yo: 220 BID ≥11 yo: 440 BID | 8 wk | Clinical improvement/resolution of symptoms | 100 | NA | 70% Still had abnormal endoscopy (loss of vascular pattern, thickened longitudinal folds), but improved histology | 18% With esophageal candidiasis, 9% (n = 1) symptomatic | 8-wk PPI trial before diagnosis. Normal 24-h continuous pH monitoring; 9 of the patients who responded clinically to FP had failed allergy testing–based diet restriction. |
| Konikoff et al, 2006 | Randomized, double- blind, placebo-controlled | Placebo (15) | >24 eos/hpf in any ×400 HPF and epithelial hyperplasia | FP (21) | 440 BID | 3 mo | Complete response: <1 eos/hpf | 50 | 9 | All FP responders: resolved distal furrowing, epithelial hyperplasia, and vomiting | Incidental esophageal candidiasis in 9% of FP pts (1/11) | Prior acid suppression therapy was not necessary for diagnosis. FP response higher in nonallergic individuals. FP response negatively correlates with patient age, height, and weight. |
| Partial response: 1–24 eos/hpf | 15 | 9 | ||||||||||
| Schaefer et al, 2008 | Randomized, comparator controlled | Prednisone 1 mg/kg/d (40) | ≥15 eos/hpf with negative pH probe studies | FP (40) | 1–10 yo: 220 QID 11–18 yo: 440 QID | 4-wk Induction | Complete histologic resolution | 50 | 81 | 97% FP group had resolution of symptoms. 100% of prednisone group had resolution of symptoms. | Incidental esophageal candidiasis in 15% of FP patients; hyperphagia, weight gain in 40% of prednisone patients. | Symptom relapse in 44% of FP patients, 45% of prednisone 12 wk after treatment stopped. |
| Improvement in biopsy grade (score based on basal cell zone % and # eos/hpf) | 94 | 94 | ||||||||||
| Dohil et al, 2010 | Randomized, double- blind, placebo-controlled | Placebo (9) | Peak eos/hpf ≥20 | OVB (15) | <5 ft Tall: 1000/d ≥5 ft Tall: 2000/d | 3 mo | Responders: <6 eos/hpf | 87 | 0 | Endoscopy score improved more in OVB vs placebo. Symptom score improved in OVB but not placebo group. | Oral candidiasis that responded to nystatin. Serum cortisol unchanged | All patients received PPI during drug period. <10 yo: Lansoprazole 15 mg BID; ≥10 yo: lansoprazole 30 mg BID. Placebo and PPI did not improve eosinophilia at any level. |
| Partial responders: 7–19 eos/hpf | 6.7 | 11 | ||||||||||
| Nonresponders ≥20 eos/hpf | 6.7 | 89 | ||||||||||
| Boldorini et al, 2013 | Prospective | NA | >15 eos/hpf | FP (34) | 750 TID | 6 wk | Responders: ≤6 eos/hpf | 74 | NA | All children had symptomatic improvement irrespective of histologic results. Responders had more severe inflammation (higher median peak eos/hpf, higher likelihood of eosinophilic microabscesses, and peak mast cells/HPF). | No adverse events seen | All children were nonresponders to PPI or 24-h pH monitoring was negative for gastroesophageal reflux. 4 Children had celiac disease, 3 were responders 1 was not. Age, weight, and height, did not affect response. |
| Borderline: 7–20 eos/hpf | 0 | |||||||||||
| Nonresponders: >20 eos/hpf | 26 |
a Drug efficacy is based on definitions specific to each study (see Primary Outcomes column).
Fluticasone
In 2002, a prospective study using swallowed FP in children cited its ease of administration, low systemic absorption, and rapid first-pass metabolism by the liver to limit systemic side effects. These children had symptoms of esophageal dysfunction (ie, chest pain, food impaction, dysphagia, feeding refusal, and vomiting), eosinophilic esophageal infiltration, normal 24-hour continuous monitoring of intraesophageal pH (pH probe), and lack of clinical response to an 8-week trial of PPI. FP dosing was age dependent, with a maximum of 880 μg/d divided twice daily. Four patients had no food allergens identified by history, radioimmunosorbent assay, or skin prick testing and were started directly on swallowed FP. Eleven patients were started on dietary restriction and nutritional counseling based on abnormal allergy testing or history; however, none of these patients had clinical improvement and 9 were subsequently treated with swallowed FP. All 13 patients who received FP had resolution of their presenting symptoms, and all 11 patients with post-treatment endoscopy showed improvement in histology with similar decreases in eosinophilia in proximal and distal esophageal biopsies.
A subsequent randomized, double-blind, placebo-controlled trial in children showed that 50% of FP-treated patients achieved complete histologic remission (≤1 eosinophil [EOS] per high-power field [HPF]) with a standard dose, regardless of patient age and/or size, of 880 μg/d divided twice daily. Patient factors predictive of histologic resolution in this study included shorter stature and younger age. Unlike the previous study, proximal esophageal biopsies were more improved than those from the distal esophagus. Another randomized controlled trial comparing swallowed FP to oral prednisone (880–1760 μg/d based on age and 1 mg/kg/d to a maximum of 30 mg twice daily, respectively) showed complete histologic resolution in 50% of patients in FP group versus 81% in prednisone group at week 4; partial improvement in histologic grade was recorded in 94% of patients in both groups. As expected, symptomatic improvement was seen more often compared with histologic reversal; 97.2% of FP patients and 100% of prednisone patients had resolution of presenting symptoms with therapy although symptoms recurred in approximately 45% of patients 12 weeks after treatment was stopped ( Fig. 1 A).
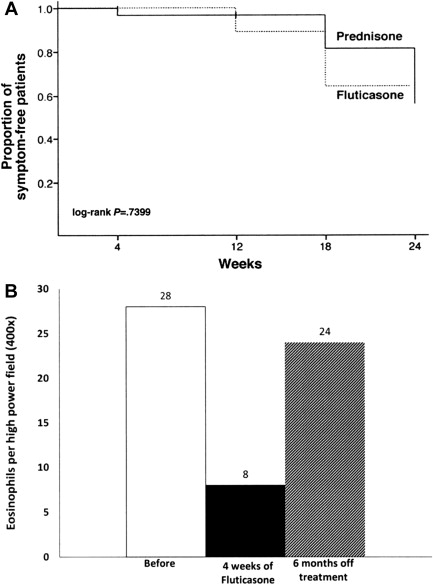
A recent prospective Italian study in children using a higher dose of FP (2250 μg/d) for 6 weeks reported higher likelihood (73.5%) in reaching post-treatment peak esophageal eosinophils of less than 6 eos/hpf and suggested that more severe esophageal inflammation (higher median peak eos/hpf, presence of eosinophilic abscesses, and peak mast cells/HPF) was associated with higher response rate to FP treatment. Age and height did not affect response in this study.
Improvement in incidental gastric eosinophilic inflammation (≥10 eos/hpf) in patients otherwise similar to EoE patients was noted with FP. Therefore, mild gastric eosinophilia should not exclude FP as a possible therapeutic option for esophageal eosinophilia.
The results of the first double-blind, randomized, placebo-controlled trial of FP (1760 μg/d) in children and adults are awaited. Further studies are needed to determine ideal dosing regimen but current recommendations are listed in Table 2 .
| Medication | Age (y) | Drug Formulation | Induction Dose | Weaning Dose | Instructions |
|---|---|---|---|---|---|
| FP | 1–10 | 110 μg/puff | 2 Puffs 4 times/d | 2 Puffs 3 times/d × 3 wk, 2 puffs 2 times/d × 3 wk, 1 puff 2 times/d × 2 wk |
|
| 11–18 | 220 μg/puff | Same as above with 220 μg/puff inhaler | Same as above with 220 μg/puff inhaler | ||
| OVB | 1–10 | 0.5 mg/2 mL budesonide respules | 1 mg Daily |
| |
| 11–18 | 0.5 mg/2 mL budesonide respules | 2 mg Daily |
Budesonide
Budesonide is another topical steroid with proved efficacy for EoE. OVB was initially developed to help patients who were developmentally unable to perform the puff and swallow technique required for FP. The first studies to evaluate its efficacy mixed aqueous budesonide (0.5 mg/2 mL suspension, Budesonide Respules [Pulmicort], Astra-Zeneca, Wilmington, DE) with sucralose (see Table 2 for recipe) to create a thickened slurry. A randomized, double-blind, placebo-controlled trial in children showed significant improvement in symptoms, endoscopic findings, and esophageal eosinophilia compared with placebo. Patients less than 1.5 meters (5 feet) tall received 1 mg daily; patients greater than or equal to 5 feet tall received 2 mg daily for 3 months. Patients in both groups also received twice-daily lansoprazole (15 mg twice daily if less than 10 years old and 30 mg twice daily if greater than 10 years old). Peak eosinophil counts in the OVB group improved from 66.7 to 4.8 eos/hpf, with significant reductions in proximal, mid-, and distal esophageal eosinophilia.
No studies to date have compared FP and OVB in children.
Ciclesonide
Two small case series report a total of 8 children treated with ciclesonide, a topical CS also used in asthma, allergic rhinitis, and allergic conjunctivitis. Six of the 8 patients showed histologic improvement; the 2 who did not respond had previous poor response to OVB as well. In asthma, inhaled ciclesonide seems to have similar effectiveness compared with inhaled FP and nebulized budesonide. Larger randomized, drug-controlled studies are needed to see if this is the case in EoE.
Topical corticosteroids for induction
In 1998, Faubion and colleagues described 4 children with eosinophilic inflammation isolated to the esophagus who improved clinically and histologically by swallowing aerosolized CSs (FP and beclomethasone) from an inhaler without use of a spacer. Over time, FP has become the topical CS used most often in EoE, although other agents are also used (discussed later). We will review prospective and randomized studies involving topical steroids used in pediatric eosinophilic esophagitis ( Table 1 ). Adult studies are discussed elsewhere in this issue.
| Study | Type of Study | Control Group (n) | Histologic Criteria | Drug (n) | Dose (μg) | Length of Treatment | Primary Outcomes | Drug Efficacy a (%) | Control Group Response (%) | Other Outcomes | Adverse Events | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teitelbaum et al, 2002 | Prospective | NA | >15 eos/hpf, superficial layering, and/or eosinophil microabscesses | FP (13) | 2–4 yo: 88 BID 5–10 yo: 220 BID ≥11 yo: 440 BID | 8 wk | Clinical improvement/resolution of symptoms | 100 | NA | 70% Still had abnormal endoscopy (loss of vascular pattern, thickened longitudinal folds), but improved histology | 18% With esophageal candidiasis, 9% (n = 1) symptomatic | 8-wk PPI trial before diagnosis. Normal 24-h continuous pH monitoring; 9 of the patients who responded clinically to FP had failed allergy testing–based diet restriction. |
| Konikoff et al, 2006 | Randomized, double- blind, placebo-controlled | Placebo (15) | >24 eos/hpf in any ×400 HPF and epithelial hyperplasia | FP (21) | 440 BID | 3 mo | Complete response: <1 eos/hpf | 50 | 9 | All FP responders: resolved distal furrowing, epithelial hyperplasia, and vomiting | Incidental esophageal candidiasis in 9% of FP pts (1/11) | Prior acid suppression therapy was not necessary for diagnosis. FP response higher in nonallergic individuals. FP response negatively correlates with patient age, height, and weight. |
| Partial response: 1–24 eos/hpf | 15 | 9 | ||||||||||
| Schaefer et al, 2008 | Randomized, comparator controlled | Prednisone 1 mg/kg/d (40) | ≥15 eos/hpf with negative pH probe studies | FP (40) | 1–10 yo: 220 QID 11–18 yo: 440 QID | 4-wk Induction | Complete histologic resolution | 50 | 81 | 97% FP group had resolution of symptoms. 100% of prednisone group had resolution of symptoms. | Incidental esophageal candidiasis in 15% of FP patients; hyperphagia, weight gain in 40% of prednisone patients. | Symptom relapse in 44% of FP patients, 45% of prednisone 12 wk after treatment stopped. |
| Improvement in biopsy grade (score based on basal cell zone % and # eos/hpf) | 94 | 94 | ||||||||||
| Dohil et al, 2010 | Randomized, double- blind, placebo-controlled | Placebo (9) | Peak eos/hpf ≥20 | OVB (15) | <5 ft Tall: 1000/d ≥5 ft Tall: 2000/d | 3 mo | Responders: <6 eos/hpf | 87 | 0 | Endoscopy score improved more in OVB vs placebo. Symptom score improved in OVB but not placebo group. | Oral candidiasis that responded to nystatin. Serum cortisol unchanged | All patients received PPI during drug period. <10 yo: Lansoprazole 15 mg BID; ≥10 yo: lansoprazole 30 mg BID. Placebo and PPI did not improve eosinophilia at any level. |
| Partial responders: 7–19 eos/hpf | 6.7 | 11 | ||||||||||
| Nonresponders ≥20 eos/hpf | 6.7 | 89 | ||||||||||
| Boldorini et al, 2013 | Prospective | NA | >15 eos/hpf | FP (34) | 750 TID | 6 wk | Responders: ≤6 eos/hpf | 74 | NA | All children had symptomatic improvement irrespective of histologic results. Responders had more severe inflammation (higher median peak eos/hpf, higher likelihood of eosinophilic microabscesses, and peak mast cells/HPF). | No adverse events seen | All children were nonresponders to PPI or 24-h pH monitoring was negative for gastroesophageal reflux. 4 Children had celiac disease, 3 were responders 1 was not. Age, weight, and height, did not affect response. |
| Borderline: 7–20 eos/hpf | 0 | |||||||||||
| Nonresponders: >20 eos/hpf | 26 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




