CHAPTER 27 Eosinophilic Disorders of the Gastrointestinal Tract
Eosinophilic inflammation of the gastrointestinal (GI) tract occurs in primary eosinophilic GI disease (EGID), as well as secondary to other diseases. The classification of primary EGID is traditionally according to the sites of inflammation in the GI tract (Table 27-1).1,2 The best characterized of these EGIDs, eosinophilic esophagitis (EE) and eosinophilic gastroenteritis (EG), affect all ages and exhibit prominent eosinophilic tissue infiltration. Other noteworthy diagnoses within the spectrum of EGIDs, such as food protein–induced enterocolitis (FPIEC) and eosinophilic proctitis (EP), are uniquely pediatric diagnoses, and the pathology may be characterized by a mixed inflammatory infiltrate including dense tissue eosinophilia. The cause of these disorders may not be yet well understood, but they all have a strong association with allergies, and many respond to nutritional management (see Chapter 9).3,4 The collaborative efforts of gastroenterologists, allergists, and immunologists have made significant advances in understanding the immunopathogenesis of EGID in recent years. The publication of guidelines for the diagnosis and management of EE also became possible through such efforts.5
Table 27-1 Proposed Classification and Differentiation of Primary and Secondary Eosinophilic Gastrointestinal Diseases
| PRIMARY EGID | SECONDARY EGID AND/OR DIFFERENTIAL DIAGNOSIS |
|---|---|
EE, eosinophilic esophagitis; EG, eosinophilic gastroenteritis; EGID, eosinophilic gastrointestinal disease; GERD, gastroesophageal reflux disease; HES, hypereosinophilic syndrome; IBD, inflammatory bowel disease.
Eosinophilic inflammation also occurs secondarily in the GI tract in inflammatory bowel disease (IBD), autoimmune diseases, reactions to medications,6,7 infections, hypereosinophilia syndrome (HES), tumors, and after solid organ transplantation.8,9 These disorders should be considered in the differential diagnosis of the primary eosinophilic diseases and are briefly reviewed in this chapter.
EOSINOPHIL: ROLE IN HEALTH AND DISEASE
The eosinophil, a bilobed nucleated granulocyte, differentiates from myeloid progenitor cells into its mature form containing brilliant birefringent cationic granules with a high affinity for the acidic dye eosin. It matures mainly under the influence of the hematopoiesis-specific transcription factors GATA-1, GATA-2, and PU.I, and c/EBP (enhancer-binding protein family).10 Cytokines, interleukin-3 (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) strongly influence further development. IL-5, in particular, plays a role in the eosinophil’s differentiation and release from the bone marrow into the peripheral circulation, where it constitutes 2% to 4% of the granulocyte pool and has a circulating half-life of only 8 to 12 hours. The eosinophil then moves into resident tissues, mainly the GI tract, thymus, hematopoietic organs, and mammary glands. In the GI tract, eosinophils survive for about 1 week and finally undergo apoptosis.11
EOSINOPHILS AND THE GASTROINTESTINAL TRACT
As noted, eosinophils spend most of their lifespan in tissues, rather than circulating. The GI tract is the main nonhematopoietic organ in which eosinophils reside in the healthy state. In the GI tract, eosinophils are not homogeneously distributed. Highest concentrations are found in the cecum, ascending colon, and appendix. The esophageal epithelium is unique in being devoid of eosinophils under noninflammatory conditions.11,12 Eosinophils are normally present in the lamina propria of the gut, but the number of eosinophils regarded as pathologic for various sites along the GI tract is debated.
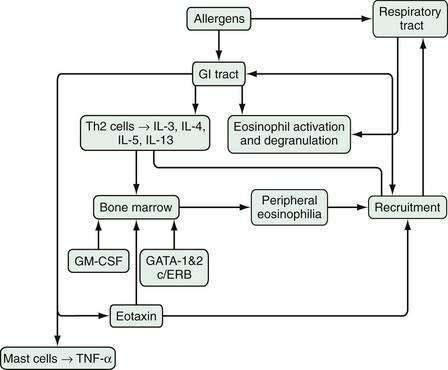
An array of stimulatory and proinflammatory factors mediate eosinophilic inflammation (Fig. 27-1). In the case of eosinophilic GI inflammation, an antigen exposure stimulates eosinophil synthesis, rolling, adhesion, diapedesis, and trafficking to the site of insult. The recruitment of eosinophils into GI segments is regulated by differential pathways involving a family of cell adhesion receptors called integrins. Investigations have shown that eosinophil movement into the small intestine and large intestine are controlled by α4β7-integrin and β2-integrin pathways, respectively.10,13,14 Eosinophils function as antigen-presenting cells and also affect the inflammatory process through specific eosinophil-derived granule proteins (EDGPs). These EDGPs include eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), eosinophil peroxidase (EPO), and major basic protein (MBP). These cationic proteins are cytotoxic to the human intestinal epithelium, possess antiviral and ribonuclease activity, and trigger degranulation of mast cells and release of cytokines (IL-1, IL-3, IL-4, IL-5, IL-13, GM-CSF, tumor necrosis factor-α [TNF-α], transforming growth factors), chemokines such as eotaxin-1 and RANTES (regulated on activation, normal T cell expressed and secreted), lipid mediators (leukotrienes, platelet-activating factor), and neuromediators (substance P, vasoactive intestinal polypeptide, nerve growth factor).15
Investigators have used various experimental models to explore the mechanisms whereby eosinophils mediate GI disease. An important puzzle includes the localization of the instigation of eosinophilic responses within the GI tract. The route of allergen exposure may determine the localization of the response. For example, oral or intragastric allergen exposure does not initiate EE but, in anesthetized mice, exposure to repeated challenges of aeroallergens induces marked EE in addition to lung eosinophilia. Interestingly, however, such aeroallergen challenge does not provoke eosinophilic inflammation in the stomach or small intestine of the mice.14,16 In human EE, therefore, sensitization likely occurs via the respiratory tract, with subsequent exposure to oral allergens leading to a hypersensitivity response and esophageal eosinophil infiltration.
Experimental studies have suggested the mechanism of this link between the lung and esophagus via T helper 2 (Th2) allergic responses in the lung and esophagus.14,16,17 Th2 cells (see Chapter 2 for more details) produce an array of cytokines, of which IL-5 is the most specific for eosinophils, inducing eosinophil growth, differentiation, activation, and survival, and enhancing responsiveness to chemoattractants such as eotaxin-1, eotaxin-2, and eotaxin-3 (eosinophil selective chemokines structurally distinguished from others on the basis of conserved cysteines). Further studies using the murine model of EE have demonstrated an important role for IL-5, IL-13, and eotaxin in this disorder.18–20 In IL-5–deficient mice, the allergen-induced EE response is ablated and, in the absence of eotaxin, it is attenuated. Furthermore, the absence of IL-5 reduces the esophageal eosinophilia induced by oral allergens after sensitization to aeroallergens, but the absence of IL-5 does not reduce intestinal eosinophilia, strongly suggesting a differential recruitment of eosinophils in EE and EG.
In humans the esophageal infiltrate in EE also includes increased numbers of T cells and mast cells and increased IL-5, TNF-α, and eotaxin.21,22 Recent evidence that IL-13 delivery into the lung induces EE further implicates Th2 cells and cytokines in the immunopathogenesis of EE.17,23 Furthermore, esophageal biopsies in EE are notable for sharing some remodeling features with airway disease in asthma, such as increased profibrotic cytokines, signaling molecules, increased vascularity, and vascular activation.24,25
In a placebo-controlled experiment using another murine model, mice challenged with oral, encapsulated ovalbumin developed peripheral blood eosinophilia and antigen-specific immunoglobulin E (IgE) and IgG1 antibodies. Their eosinophil-predominant cellular infiltrate was largely localized in the lamina propria throughout the small intestine but was also present in the esophagus, stomach, and Peyer’s patches. The mice developed gastromegaly, dysmotility, and cachexia, thought to be correlates of human EGID.26 In a mouse model of the homozygous lyp gene mutation (lyp protects against lymphocyte apoptosis), increased levels of Th2 cytokines and IgE are observed in association with clinical features of bloating, intestinal distention, wasting, splenomegaly, and increased intestinal eosinophilia.27
Some patients with EE have also been characterized as having a unique genomic transcript comprised of an increased expression of the gene encoding eosinophil-specific chemoattractant eotaxin-3 compared with healthy patients.18,28
CLINICAL ENTITIES
EOSINOPHILIC ESOPHAGITIS
In the esophagus, attention to eosinophilic infiltration has focused on the epithelium, rather than on the lamina propria. This squamous epithelium normally is devoid of eosinophils, but various disorders cause eosinophils to infiltrate the esophageal epithelium. In general, such esophageal eosinophilic infiltration is considered to be secondary to an extraesophageal cause (e.g., parasitic infections, autoimmune diseases, vasculitis, HES, medications) or an esophageal cause (e.g., gastroesophageal reflux disease [GERD])29 or to be primary EE. Primary EE may be divided into allergic or idiopathic cases, depending on whether identifiable allergens play a role (see Table 27-1). Occasional patients presenting with apparent EE have marked eosinophilic inflammation of other segments of the GI tract, and designation as EE secondary to EG or as a form of primary EE is a matter of semantics.
Primary EE, rarely diagnosed until the mid-1990s, currently represents an important esophageal disorder, particularly in children, but increasingly in adults. The emergence of this disease has paralleled the increasing incidence of allergies and asthma. Whether the increasing number of cases of EE is to the result of increased recognition of EE or a truly increased incidence of EE is still debatable.30–32 Although some of the previous lack of recognition in adults may have resulted from failure to biopsy intact-appearing esophageal mucosa, the actual prevalence of EE in adults has increased, as evident in some recent reviews.33–39 Similarly, although the routine biopsying of even normal-appearing mucosa by pediatric gastroenterologists may account for some of the predominance of EE in school-age children, it is likely that this age group currently does experience more EE, with another peak in young adulthood.40,41 Like allergic disorders, EE’s prevalence also varies markedly in different locales and perhaps in different seasons.42 The last 10 years or so have seen a dramatic increase in the diagnosis of EE around the world; this may be the result of both improved recognition and an actual increase in new cases akin to other allergic disorders. The incidence of pediatric EE in Australia increased from 0.05 to 0.89/10,000 during 1995 to 2004, according to a retrospective review.43 In the U.S. Northeast, a twofold increase in incidence was observed in a four-year period at Children’s Hospital of Philadelphia.44 In the U.S. Midwest, a fourfold increase in prevalence was reported.45 The prevalence of EE was reported as 15/100,000 inhabitants in one region of Switzerland, perhaps an underestimation because of limited expertise in its diagnosis.46 Markedly more prevalent in males, the disorder occurs in females as well. Duration of symptoms before diagnosis may vary from just a few days in those presenting with sudden episodes of food impaction to many years in those with GERD-like symptoms.4,47 Like allergies in general, EE clusters in families and an autosomal dominant pattern of inheritance have been proposed on the basis of the 10% rate of familial clustering.48 A personal history of atopy in association with EE in children and adults occurs in 50% to 80% cases, with food allergy accounting for an allergic diathesis in up to 90% children; 39% report a family history of allergies.49–52 In one series, symptoms of chronic respiratory disease were seen in 62% of patients.40 In support of the theory that aeroallergens promote the disease are cases of EE with symptomatic and biopsy-proven exacerbations during pollen season and resolution during winter months.53,54
The first report of EE in 1978 and subsequent reports have characterized the phenotype of EE.35,40,44,55,56 Symptoms of EE are similar to those of GERD, but respond poorly to antireflux medical and surgical therapy.57 Whereas younger children with EE frequently present with GERD-like symptoms, feeding problems, and abdominal pain,58,59 adolescents and adults present with obstructive presentations such as dysphagia or food impactions, with or without strictures.36,47 The degree to which these presentations represent actual structural obstruction versus dysmotility is unclear and appears to vary among patients.60 Esophageal biopsy of patients without any GI symptoms, such as some patients presenting for evaluation of respiratory symptoms, has disclosed unsuspected EE.37,61
The brief history of recognition of EE has prevented clear definition of its natural history, but EE appears to be a chronic disease with a waxing and waning course, as suggested by a noteworthy relapse rate of 80% in an eight-year follow-up of children with EE and similarly high rate of recurrent symptoms and chronic therapy in adults.62,63 It is thought that the esophageal wall is fragile and weakened in patients with chronic EE, thus predisposing to endoscopy-related and spontaneous perforation.36,64,65 Another study of the long-term (mean, 7.2 years) follow-up of EE in adults has reported persistence of eosinophilic inflammatory infiltrate (albeit significantly reduced compared with baseline) and dysphagia and a high rate of subepithelial fibrosis and sclerosis, perhaps the mechanistic link to esophageal strictures in EE.41 Although not supported by any published data, EE patients are also considered to have a low quality of life. This information raises questions regarding the pros and cons of treatment for asymptomatic patients with EE. The current literature does not clearly identify any malignant potential of the disease.66,67
EOSINOPHILIC GASTROENTERITIS
Eosinophilic gastroenteritis is a heterogeneous disorder affecting children and adults characterized by the presence of an intense eosinophilic infiltrate on histopathology of one or multiple segments, from the esophagus to the rectum.68 These eosinophilic infiltrates not only may involve various sites down the length of the GI tract, but also may occupy various sites through the depth of the wall. These inconsistencies from case to case promote unpredictability in presenting symptoms, which range from pain to dysmotility, bleeding, obstruction, or ascites.69–72 Since the initial report of EG seven decades ago, reports of EG have emerged from different parts of the world, including North America, Europe, Australia, and Asia.72–77 These reports provide important information regarding epidemiology, disease characteristics, and management.
The diagnosis of EG is rare, with an approximate incidence of 1/100,000, but it is also possible that physicians make the diagnosis of EG infrequently because of the inaccessibility of much of the length of the small bowel and of the deeper layers of the luminal wall. Therefore, the literature on EG has been somewhat anecdotal. Retrospective review of an 18-year period at a hospital in China identified 15 patients with EG, including 2 children.74 Histologic evaluation established the diagnosis in 13, and radiologic findings, combined with eosinophilic ascites, suggested it in the remaining 2 patients.
In one of the largest series, Talley and colleagues compared laboratory and clinical data on 40 adults diagnosed with EG during a 30-year period with data on 10 other patients with similar GI symptoms but no tissue eosinophilia.73 EG is most commonly diagnosed between the second and sixth decades of life, and is rarely diagnosed in infants.1,74,78 Unlike EE, which favors males, EG does not appear to manifest a significant gender disparity. It is associated with asthma and allergies in 40% to 50% of cases.79,80 Peripheral eosinophilia may be seen in up to 80% of cases, but is not a prerequisite for diagnosis.81
In published reports, the stomach (26% to 81%) and small intestine (28% to 100%) are the predominantly affected areas, but the esophagus, large intestine, and rectum may be affected as well.1,80 The depth of infiltration varies and leads to the broad spectrum of clinical manifestations in patients with EG. The classification of EG on the basis of depth of eosinophilic infiltration proposed by Klein and associates is currently the one most cited in publications.82
Mucosal Eosinophilic Gastroenteritis
Those with mucosal inflammation usually present with common, albeit nonspecific, complaints of abdominal pain, nausea, vomiting, diarrhea, fecal occult blood loss, anemia, or protein-losing enteropathy. Because of the nonspecific nature, these clinical presentations may be confused with irritable bowel syndrome, dyspepsia, peptic ulcer, pancreatitis, acute appendicitis, or IBD.83 Eosinophilic enteritis presenting in an adult as intussusception, and treated effectively with the nonsurgical option of prednisone, has been observed.76 Frequently, atopy and high IgE levels coexist.73,74 An example of eosinophilic gastritis is shown later (see Fig. 51-8B).
Muscular Eosinophilic Gastroenteritis
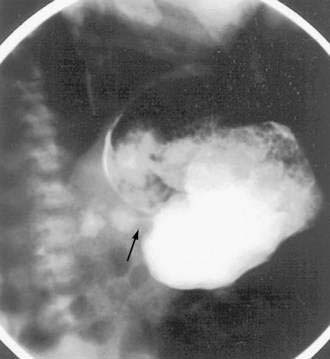
Signs and symptoms of gastric outlet and intestinal obstruction are common in those with muscular EG.84,85 The presentation of gastric outlet obstruction mimicking hypertrophic pyloric stenosis has been reported in infancy and adulthood.86,87 A hypoallergenic diet has been shown to alleviate the condition in infants (Fig. 27-2). Enteric strictures are rare, but can occur in children and adults with EG.88 As a sort of amalgam between the mucosal and muscular forms, one patient was reported to have eosinophilic inflammation of myenteric plexus and the lamina propria on colonic biopsies, producing functional intestinal obstruction.89
Serosal Eosinophilic Gastroenteritis
Involvement of the serosal layer occurs in 10% of cases of EG and typically presents as ascites. The serosal form of EG, compared with other types, is reported to be associated with significant bloating, a higher level of peripheral eosinophilia, and a better response to glucocorticoid therapy.73,90,91
The natural history of EG remains somewhat vague, although recent pediatric data has supported a protracted course, and hence the need for long-term treatment strategies, including dietary restrictions and repeated use of glucocorticoids.78
FOOD PROTEIN–INDUCED ENTEROCOLITIS
Enterocolitis signifies an inflammatory process involving the small and large intestines. FPIEC represents a symptom complex of severe vomiting and diarrhea that usually presents in infancy as a reaction to ingested proteins. The onset of symptoms is in the first few weeks of life. The trigger is most often ingestion of cow’s milk protein-based formula, but approximately half of infants also react to soy. Other food proteins, including rice, oats, and chicken, have also been implicated in individual cases.92–94 The responsible dietary antigens in the maternal diet are thought to sensitize via breast milk. The profuse, often bloody and mucoid, diarrhea is associated with weight loss and malnutrition in an ill-appearing patient. An association with methemoglobinemia noted in several cases was attributed to increased heme oxidation caused by an elevation of nitrite levels in the intestine in severe intestinal inflammation.95,96
Typically, FPIEC is caused by non-IgE mediated delayed food protein hypersensitivity, so allergy testing with skin prick tests (SPTs) and RASTs are negative; patch tests have not been adequately studied in this diagnosis.97,98 Patients lack evidence for other causes of eosinophilia, such as infections, inflammatory bowel disease, and ischemia. The diagnosis rests on clinical criteria and resolution after elimination of the causal milk and soy proteins from the diet. Most infants do well when their milk is changed to an extremely hydrolyzed formula that digests the intact proteins into small polypeptides of sizes that do not engender the hypersensitivity response. Up to 90% of such infants can tolerate milk by 3 years of age. Any milk challenge should be performed under medical supervision because of the risk of serious reactions leading to shock. Criteria for a failed challenge include vomiting, diarrhea, gross or occult fecal blood, fecal leukocytes, fecal eosinophils, and elevated white blood cell count.
EOSINOPHILIC PROCTITIS
EP uniquely affects children younger than 2 years. These children present with bloody stools, either alone or in association with diarrhea. The condition has been reported in infants receiving cow’s milk and soy protein–based formulas, as well as in exclusively breast-fed infants. A few infants suffer from eczema, but otherwise these children lack any systemic symptoms. In contrast to children with enterocolitis, these babies generally appear well. In a review, all 95 exclusively breast-fed infants evaluated for proctitis over 20 years had blood-tinged stools, and one third of them were observed to have painful defecation and eczema.99 The diagnosis may be confused with colic or GERD in infants who also present with irritability or vomiting. In a prospective study, 18% of 40 breast-fed infants presenting with rectal bleeding were diagnosed to have cow’s milk allergy on the basis of milk elimination and provocation; association with food-specific IgE and positive SPTs was uncommonly noted.100
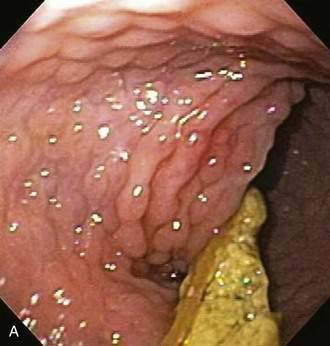
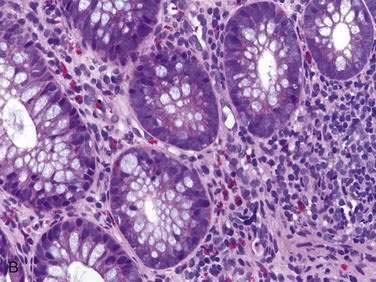
Endoscopic examination reveals focal rectal mucosal erythema, erosions, and lymphoid nodular hyperplasia (Fig. 27-3A). Histopathologic examination shows prominent eosinophil infiltration in the mucosa and lamina propria, at least 6 eosinophils/high-power field, and/or eosinophils invading crypts or the muscularis mucosae (see Fig. 27-3B).101 There is an excellent response to elimination diets using hydrolyzed or elemental formulas; by 1 year of age most infants can tolerate a rechallenge with the offending food proteins.
EVALUATION
LABORATORY EVALUATION
Peripheral eosinophilia in the context of GI symptoms is a useful clue to EGID, but the absence of eosinophilia does not exclude these diagnoses.102 It is important to note that circulating eosinophils represent a balance between bone marrow production and tissue infiltration. Moreover, the frequently observed fluctuations in peripheral eosinophil concentrations may be caused by the effects of the circadian rhythm.103 To exclude important secondary causes for GI eosinophilia, evaluation should generally include stool or duodenal aspirate for ova and parasites. In those with ascites, paracentesis may provide the only clue to the diagnosis in the form of ascitic fluid eosinophilia. Hopefully, our increasing knowledge will soon allow simple, reliable, and relatively noninvasive testing involving markers of active eosinophil inflammation (e.g., fecal ECP) for monitoring disease course and response to treatment.104
ALLERGY EVALUATION
Immunologic evidence of underlying allergy is usually lacking in most EGIDs, except those mediated by IgE antibody and typically presenting with immediate reactions or accompanied by eczema or asthma (see Chapter 9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree