Chapter 41 Endoscopic Ultrasound of Pancreatic and Biliary Diseases
Introduction
Endoscopic ultrasound (EUS) is a powerful technique that has changed the clinical approach to biliary and pancreatic disease. Because it provides detailed images of the extrahepatic biliary tree and pancreas with very little risk to the patient, EUS is useful in the evaluation of obstructive jaundice,1 biliary or pancreatic ductal dilation,2 pancreatic masses, and pancreatitis.3 EUS and endoscopic retrograde cholangiopancreatography (ERCP) can be performed under the same sedation, with EUS identifying patients likely to benefit from therapeutic ERCP. EUS-guided therapeutic interventions have an evolving role in selected patients, including celiac plexus block (CPB),4 drainage of pancreas fluid collections, and EUS-guided drainage of inaccessible biliary and pancreatic ducts.5 EUS is an increasingly important tool for biliary and pancreatic endoscopy. This chapter discusses the use of EUS for diagnosis of common biliary and pancreatic diseases and emerging methods of EUS-guided pancreaticobiliary therapy.![]() The included video clips on the accompanying website show station-based techniques for performance of biliary and pancreatic EUS. The techniques of EUS-guided fine needle aspiration (FNA) and tissue biopsy are presented in a subsequent chapter.
The included video clips on the accompanying website show station-based techniques for performance of biliary and pancreatic EUS. The techniques of EUS-guided fine needle aspiration (FNA) and tissue biopsy are presented in a subsequent chapter.
Gallbladder Stones, Sludge, and Polyps
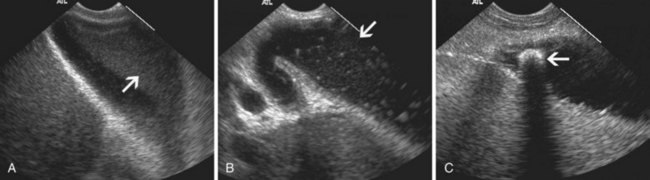
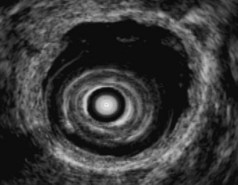
EUS is useful for diagnosis of gallbladder sludge or stones missed by transabdominal ultrasound and is more sensitive than bile microscopy in such patients (Fig. 41.1).6,7 It may be especially useful in obese patients and patients with stones in the gallbladder neck, settings in which transabdominal ultrasound is less sensitive for diagnosis. Sludge is visualized as echogenic, nonshadowing, layering material. It should not be confused with gain artifact or “ring-down” artifact, circular bright lines parallel to the transducer seen with mechanical radial scopes. A clear-cut sludge-bile interface is helpful for diagnosis of sludge. Cholesterol crystals have straight edges and are highly echogenic; they appear as bright flecks in bile, sometimes casting “comet tails.” Calcium bilirubinate granules are rounded and much less echogenic and can be missed by EUS unless they are present in sufficient quantity to form layering sludge.
EUS has been used for differential diagnosis of gallbladder polyps. The best clinical studies have been reported from Asia, and their applicability to Western populations has not been well studied. Most gallbladder polyps are readily imaged, although the fundus and cap of the gallbladder may be difficult or impossible to visualize in some patients. Adherent sludge can mimic a gallbladder polyp, but sludge can usually be distinguished from polyp by gently shaking the patient’s abdomen or turning the patient to the right decubitus position during EUS to determine whether the lesion moves (Fig. 41.2). The differential diagnosis of gallbladder polyps is presented in Table 41.1. The size of gallbladder polyps is the most important consideration in differential diagnosis. In Asian populations, neoplasm is very unlikely in polyps with a diameter of 5 mm or less but is usually present in polyps greater than 15 mm in diameter.8–10
Table 41.1 Differential Diagnosis of Gallbladder Polyps
| Nonneoplastic | Neoplastic |
|---|---|
| Cholesterol | Adenoma |
| Hyperplastic | Adenocarcinoma |
| Inflammatory | Adenosquamous carcinoma |
| Fibrous | Neuroendocrine tumor |
| Adenomyomatosis |
Particular echo features that predict the type of polyp have been identified. Neoplastic polyps may contain hypoechoic foci,11 whereas cholesterol polyps often contain bright, echogenic spots or show “comet tail” artifacts caused by cholesterol crystals in the lesion. Adenomyomatosis typically contains multiple small cystic or anechoic spaces and may show evidence of cholesterol deposits in the lesion. When these distinctive findings are seen, it may be reasonable to follow larger gallbladder polyps, at least polyps less than 20 mm in diameter,10 although neoplastic polyps having EUS features of cholesterolosis or adenomyomatosis have been reported.12 Neoplasm should be considered when the characteristic findings of a nonneoplastic lesion are absent and the lesion is greater than 5 mm, even in lesions confined to the mucosa. Loss of gallbladder wall architecture is suggestive of an invasive cancer.13
Bile Duct Stones
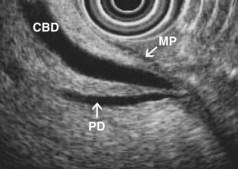
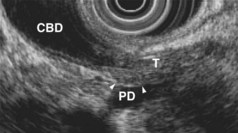
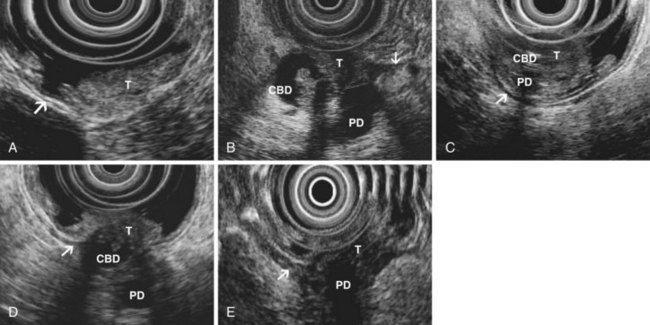
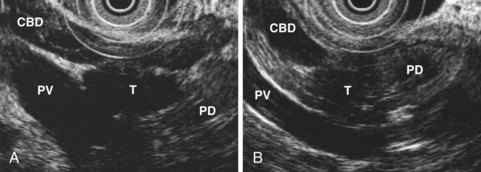
EUS is highly accurate for diagnosis of choledocholithiasis. EUS is especially useful in patients with a low or intermediate risk of bile duct stones, refining the use of ERCP and decreasing the overall risks of an endoscopic approach. The accuracy of EUS for diagnosis of bile duct stones and sludge relies on both endoscopic and patient factors. The common duct is best visualized from the duodenal bulb, where it can be followed from the common hepatic duct to the periampullary region. The biliary confluence is sometimes visualized from the bulb, more often with linear array echoendoscopes. The bile duct is distinguished from adjacent vessels by identifying its convergence with the pancreatic duct as both ducts taper into the duodenal wall—the “stack” sign (Fig. 41.3). The cystic duct insertion is another useful landmark. In addition, the bile duct wall has an inner hypoechoic mucosal layer that is not present in adjacent vessels. Imaging should also be performed with the endoscope opposite the ampulla in the second duodenum for detection of stones in the ampulla or periampullary bile duct.
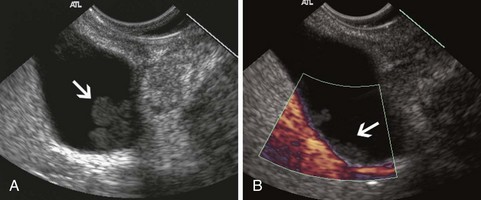
Stones are identified as echogenic structures casting dark acoustic shadows (Fig. 41.4). Air bubbles in the duct also appear as echogenic, rounded structures but cast hyperechoic acoustic reverberations instead of shadows. Sludge or cholesterol crystals can be visualized in the bile duct much as they are visualized in the gallbladder (see Fig. 41.1). Diagnosis of ductal stones is easiest when small stones are present in a dilated duct—the very situation in which cholangiography can miss stones. Conversely, ultrasound diagnosis may be challenging when a diminutive duct is present or when the common duct is completely filled with stones, obliterating a visible ductal lumen. Care should be taken to visualize the entire bile duct, not skipping over portions. It is important to recognize a technically inadequate or incomplete EUS examination and to consider other imaging tests rather than concluding that no stones are present.
EUS has been compared with ERCP for diagnosis of bile duct stones. Prat and colleagues14 performed EUS and ERCP in 119 patients with suspected bile duct stones, performing sphincterotomy and balloon sweeps of the bile duct in all subjects to obtain an independent “gold standard.” The sensitivity and specificity of EUS were 93% and 97% compared with 89% and 100% for ERCP. This study suggested that radial EUS was more sensitive than cholangiography for diagnosis of stones. A study of similar design using linear array echoendoscopes reported that the sensitivity and specificity of EUS were both 93%.15 Magnetic resonance cholangiopancreatography (MRCP) and EUS are both good tests for detection of bile duct stones, with roughly equivalent overall accuracy.16 EUS seems to be more accurate for diagnosis of stones less than 5 mm in diameter and may be preferable for diagnosis of ampullary stones,17 whereas intrahepatic duct stones not visualized by EUS can be diagnosed on MRCP.
EUS can be used as the sole imaging study to exclude choledocholithiasis before laparoscopic cholecystectomy. When EUS showed no bile duct stones, recurrent symptoms owing to ductal stones did not occur during almost 3 years of follow-up in a European cohort.18 Several prospective randomized trials compared clinical outcomes when either EUS or ERCP was used to evaluate the bile duct in patients at intermediate risk for bile duct stones, such as patients with uncomplicated biliary pancreatitis or elevated serum liver tests.19–22 Taken together, these studies show higher diagnostic accuracy, fewer overall complications, and less resource use in patients evaluated with EUS.17 Most patients enrolled in the EUS arm of these studies did not have bile duct stones and did not require ERCP; patients who did have ductal stones usually underwent ERCP immediately after EUS, under the same sedation. EUS has emerged as a preferred alternative to ERCP in patients at intermediate risk of bile duct stones.
Biliary intraductal ultrasound (IDUS) is also accurate for diagnosis of bile duct stones and sludge. IDUS requires deep cannulation of the bile duct with the intraductal probe, which can be passed over a guidewire without sphincterotomy. Most investigators have performed IDUS after obtaining a cholangiogram during ERCP. To minimize trauma to the probe and extend its useful life, the operator should use as little elevator as possible and image only during slow probe withdrawal. Because IDUS uses a high-frequency probe placed directly in the duct, it is probably the best available imaging technique for diagnosis of small stones and ductal sludge. In one direct comparison of cholangiography and IDUS, the sensitivity of IDUS for stones was 97% compared with 81% for ERCP.23 Because it is performed during ERCP, IDUS is probably best used to clarify diagnosis in patients with equivocal findings at cholangiography, such as small filling defects, possible air bubbles or polyps in the bile duct, or a dilated bile duct. IDUS improves diagnostic accuracy in about one-third of these patients.24 The need to diagnose and treat small (<5 mm) stones detected only with IDUS has been questioned, however, because such stones often pass spontaneously.25,26
Choledochoscopy is also useful for diagnosis of ductal stones; direct comparisons with IDUS have not been reported. Direct cannulation of the papilla via a linear array echoendoscope, with EUS confirmation of deep bile duct cannulation and subsequent sphincterotomy, is technically feasible and seems to have similar efficacy and complications as standard ERCP for sphincterotomy and stone extraction in patients with a solitary small bile duct stone.27 This technique may be desirable in pregnant patients because it avoids use of fluoroscopy.
Bile Duct Strictures and Cholangiocarcinoma
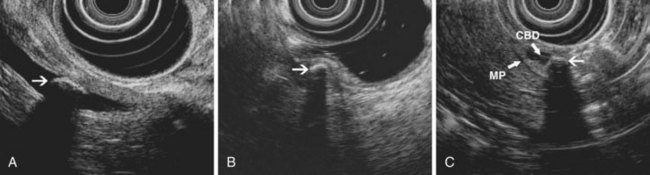
Biliary strictures may be of indeterminate etiology, typically when cross-sectional imaging and intraductal biopsy specimens and brushings obtained during ERCP are nondiagnostic. The cholangiographic appearance of a stricture and the patient’s clinical history traditionally determined whether unexplained bile duct strictures should be resected on suspicion of malignancy. EUS and IDUS may be used to evaluate biliary strictures and may aid clinical decision making by suggesting a benign or malignant process. EUS can also be used for local staging of malignant biliary strictures. The bile duct wall appears to have two or three layers on EUS and IDUS (Fig. 41.5). An internal, hyperechoic layer representing an interface echo is sometimes seen. Deep to this layer is a hypoechoic layer corresponding to the mucosa, subepithelial connective tissue, muscularis propria, and the fibrous layer of the subserosa. The amount of muscularis varies, with little or no muscularis propria in the proximal bile duct. Deep to this hypoechoic layer is an outer hyperechoic layer formed by the adipose layer of the subserosa, the serosa, and the interface with surrounding tissue.28 The normal bile duct wall is less than 1 mm thick on EUS,29 although the presence of a stent or drain in the duct may lead to thickening of the wall up to 2.8 mm.30 The bile duct wall layers can be identified with either EUS or IDUS. IDUS probes can be passed into the central intrahepatic ducts, visualizing portions of the biliary tree not usually accessible to transduodenal EUS; IDUS also provides high-resolution images of the bile duct wall and adjacent vessels and tissue. EUS with a dedicated echoendoscope can image the extrahepatic biliary tree, including Klatskin’s tumors,31 and its deeper depth of penetration permits a thorough assessment of the gallbladder, pancreatic head, and regional nodes. The two techniques are complementary.
EUS has been used for differential diagnosis of unexplained bile duct strictures. During IDUS, malignant strictures typically appear hypoechoic with a thickened wall and irregular margins, whereas postoperative strictures are usually relatively hyperechoic with smooth edges.32 Both primary sclerosing cholangitis and IgG4-associated cholangitis can mimic malignant strictures on IDUS. Studies have shown IDUS and EUS to be more accurate than ERCP and intraductal tissue sampling for diagnosis of malignant bile duct strictures,33–35 although these were retrospective studies that included few or no patients with primary sclerosing cholangitis or IgG4-associated cholangitis. In a large prospective study, IDUS was as useful as advanced cytology techniques (including fluorescent in situ hybridization) for diagnosis of indeterminate strictures.36
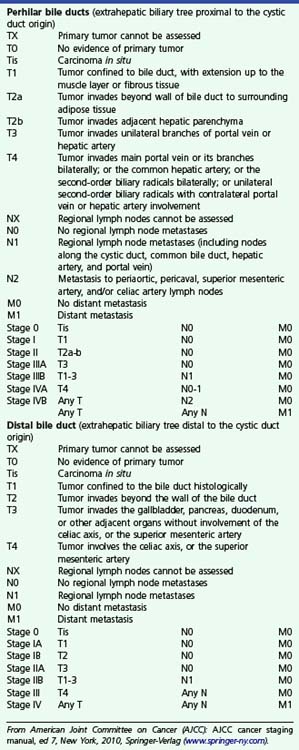
The relative utility of IDUS and direct cholangioscopy has not been widely studied, but in one report percutaneous cholangioscopy with directed biopsy was more accurate than IDUS.37 The current TNM (primary tumor, regional nodes, metastases) staging system for carcinoma of the extrahepatic bile ducts is presented in Box 41.1. Both EUS and IDUS have been used to stage cholangiocarcinoma. The two techniques have similar accuracy of about 80% for T stage, differentiating T1 lesions confined to the bile duct wall (involving the hypoechoic layer) from T2 lesions invading beyond the bile duct wall (with disruption of the outer hyperechoic layer) (Fig. 41.6).28
Box 41.1
Staging of Extrahepatic Cholangiocarcinoma
From American Joint Committee on Cancer (AJCC): AJCC cancer staging manual, ed 7, New York, 2010, Springer-Verlag (www.springer-ny.com).
IDUS is more useful than EUS for lesions of the proximal biliary tree. IDUS has also been used to estimate the longitudinal extent of cholangiocarcinoma because cholangiography is well known to underestimate the longitudinal extent of ductal involvement. Nonspecific thickening of the bile duct wall owing to the presence of a stent or drain limits the value of IDUS in patients who have had a drain placed.38 Intravenous administration of ultrasound contrast medium may improve the specificity of IDUS for malignancy, showing hyperperfusion of inflammatory lesions and hypoperfusion of tumor,39 but confirmatory studies have not been reported. IDUS is probably a sensitive modality for diagnosis of early cholangiocarcinoma in choledochal cysts. The technique should be considered in adult patients with choledochal cysts, especially if surgical resection of the cyst is not otherwise planned.
Ampulla of Vater
The submucosal apparatus of the papilla can be visualized as a round hypoechoic structure in duodenal submucosa composed of the sphincter of Oddi and the intramural ducts. The normal submucosal mound of the papilla is usually less than 6 mm in transverse cross-sectional diameter. The lumens of the bile duct and pancreatic duct are usually not visible within the papilla; they generally taper and disappear from view as they reach the duodenal wall. The finding of a visible ductal lumen in the papilla suggests obstruction of the papilla by a stone (see Fig. 41.4), stenosis, or tumor, but a ductal lumen can also be seen in choledochocele and intraductal papillary mucinous neoplasm (IPMN). IDUS has been used to study the ampulla and may aid in the local staging of some ampullary tumors. It identifies the sphincter mechanism and permits accurate measurement of its length. Ultrasound features do not distinguish normal from hypertensive sphincters.40
Ampullary Neoplasms
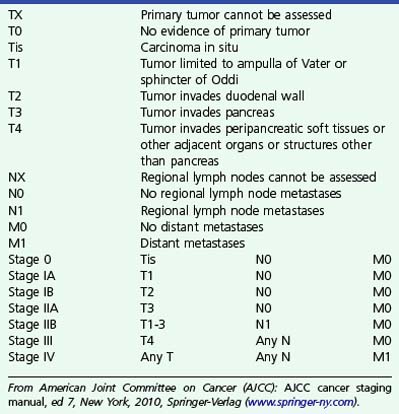
The TNM staging of ampullary cancers is presented in Box 41.2 and illustrated in Fig. 41.7. T1 carcinoma may be limited to the mucosal surfaces of the ampulla and intraampullary ducts but may also involve the sphincter mechanism of the ampulla. The presence of an irregular outer edge of the submucosal ampullary apparatus suggests a T2 lesion invading the duodenal submucosa or muscularis propria. T3 cancers invade the pancreas, extending either through the duodenal wall or directly from the periampullary ducts. A T4 tumor extends into peripancreatic soft tissue or other adjacent structures. Regional lymph nodes include not only nodes adjacent to pancreatic head but also porta hepatic and celiac nodes.
Box 41.2
Staging of Ampullary Carcinoma
From American Joint Committee on Cancer (AJCC): AJCC cancer staging manual, ed 7, New York, 2010, Springer-Verlag (www.springer-ny.com).
In one large series, EUS accuracy for T staging of ampullary malignancies was 78%.41 Adenomas were considered T1 lesions, highlighting the difficulty in distinguishing adenoma from T1 cancer with EUS. Most errors in staging involved overstaging of T2 lesions or understaging of T3 lesions because of difficulty in assessing the presence of invasion into the pancreas. The presence of peritumoral pancreatitis and edema and shadowing and tissue thickening secondary to an indwelling biliary stent were the major factors limiting the accuracy of EUS. Tumor may be difficult to distinguish from the normally hypoechoic ventral pancreas, and invasion of the duodenal muscularis propria may be difficult to detect because in normal persons the muscularis propria is interrupted by the ducts as they cross into the papilla. Despite these limitations, EUS is more accurate than computed tomography (CT) or magnetic resonance imaging (MRI).41–43
IDUS is probably more accurate that transduodenal EUS for T staging of ampullary neoplasms. In one large series, IDUS had an overall accuracy of 89%.42 IDUS visualized small tumors missed by EUS and was more accurate than endoscopic biopsies for diagnosis of ampullary neoplasm. IDUS was also accurate for differentiation of adenoma from T1 carcinoma. These results were achieved by experienced endosonographers, using IDUS at the patient’s initial ERCP and before sphincterotomy, stent placement, or biopsy—an optimal algorithm for tumor imaging but difficult to replicate in most EUS referral centers.
Acute Pancreatitis
One prospective trial investigating EUS in gallstone pancreatitis reported that it was accurate for diagnosis of gallbladder and ductal stones and predicted longer hospital stay in patients found to have peripancreatic fluid by EUS.44 In another large series in which ERCP was used selectively, on the basis of EUS findings, patient outcomes were good, and recurrent biliary pancreatitis was uncommon.45 EUS is also a useful tool in the evaluation of idiopathic pancreatitis, showing abnormalities in most patients.3,46,47 Findings include missed biliary stones or sludge (see Fig. 41.1), chronic pancreatitis, pancreas divisum, pancreatic or ampullary malignancy, and pancreatic duct stones. EUS does not diagnose pancreatic sphincter dysfunction but may nevertheless supplant ERCP by diagnosing or excluding previously unsuspected gallbladder pathology, chronic pancreatitis, or pancreatic malignancy. EUS and MRCP seemed to have similar utility in one study.48
Chronic Pancreatitis
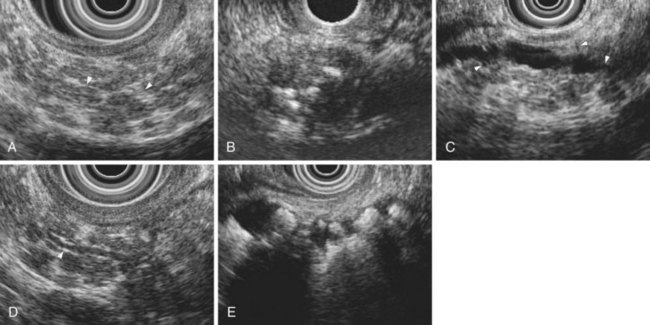
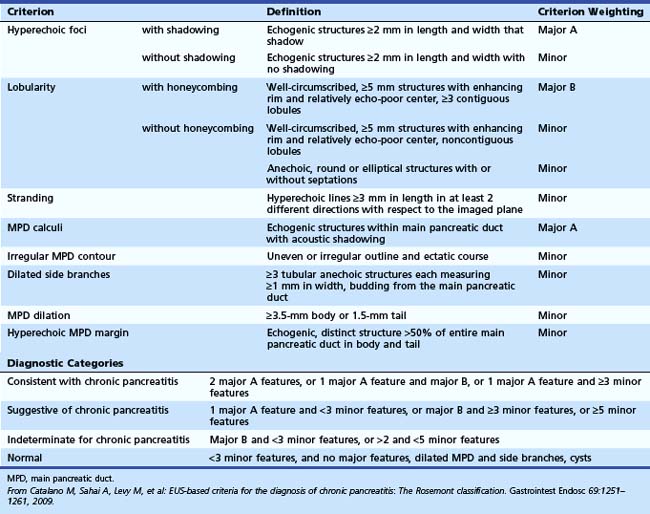
The traditional EUS features of chronic pancreatitis are listed in Table 41.2 and illustrated in Fig. 41.8. This list of consensus criteria uses minimal standard terminology adopted by an international working group,49 and good interobserver agreement has been shown for these criteria among experienced American endosonographers.50 Investigators have also described other features not included in this list, including honeycombing (in which hyperechoic strands form a honeycomb pattern), heterogeneous echotexture, focal areas of hypoechogenicity, tortuous pancreatic duct, thickened pancreatic duct wall, and narrowing of the main pancreatic duct. The traditional EUS approach to diagnosis of chronic pancreatitis gives each feature equal weight and sums the number of features present.
Table 41.2 Traditional Endoscopic Ultrasound Features of Chronic Pancreatitis
| Parenchymal Features | Ductal Features |
|---|---|
| Hyperechoic strands | Stones |
| Hyperechoic foci | Main duct irregularity |
| Lobularity | Hyperechoic main duct |
| Cysts | Visible side branches |
| Main duct dilation |
From The International Working Group for Minimal Standard Terminology in Gastrointestinal Endosonography: Minimal standard terminology in gastrointestinal endosonography. Dig Endosc 10:159–184, 1998.
The Rosemont criteria, proposed in 2009, offer an alternative approach to diagnosis based on major and minor criteria (Table 41.3).51 In one study, the Rosemont criteria resulted in improved specificity and decreased sensitivity, although these changes were not statistically significant.52 Definitions vary for some criteria. Hyperechoic foci have been defined as greater than 3 mm by some investigators53 but as 1 to 2 mm by most others.54,55 Main pancreatic duct dilation has been variably defined, often as a diameter of greater than 2 mm in the body or greater than 1 mm in the tail.50 The Rosemont criteria offer semiquantitative definitions for many criteria (see Table 41.3). Criteria have been considered abnormal when visualized at either 12 MHz or 7.5 MHz by some investigators but at only 7.5 MHz by others. Findings must be interpreted with considerable caution when imaging the pancreatic head because some features (e.g., hyperechoic strands and visible side branches) are often seen in the normal pancreatic head, whereas others (e.g., cysts and stones) are not. Diagnosis is best made based on features seen in the pancreatic body and tail. Some investigators have seen visible duct side branches in the normal pancreatic body.53
There are caveats regarding the specificity of EUS criteria for diagnosis of pancreatitis. EUS features of chronic pancreatitis have been reported in members of pancreatic cancer kindreds, in whom lobularity may correlate with the presence of pancreatic intraepithelial neoplasia in pancreatic branch ducts.56–58 Focal areas of pancreatic hypoechogenicity can be due to focal inflammation but may also be due to neoplasm (Fig. 41.9). Acute pancreatitis may cause decreased parenchymal echogenicity (owing to edema), accentuating the echogenicity of the pancreatic duct wall and the interlobular septa of the pancreas. For diagnosis of chronic pancreatitis, EUS should be performed after an acute episode of pancreatitis has resolved. Finally, ductal dilation and pancreatic fibrosis occur in older persons without clinical pancreatic disease.
Some EUS findings may be attributable to the effects of age, cigarette smoking, and alcohol on the pancreas rather than chronic pancreatitis. Although studies in normal volunteers have generally shown no parenchymal EUS abnormalities in young, asymptomatic individuals who do not use alcohol,53,55,59 older patients with no history of pancreatic disease undergoing EUS for other indications had on average two EUS features of chronic pancreatitis.60 Autopsy data show that most alcoholic cirrhotics without a clinical history of pancreatic disease have pancreatic fibrosis.61,62 EUS findings are strongly correlated with extent of ethanol ingestion; also, cigarette smoking correlates with increased number of EUS criteria.63
The accuracy of EUS for diagnosis of “early” or “minimal change” chronic pancreatitis is debated, and EUS has been compared with pancreatography, functional tests, and histology. Early studies comparing EUS with pancreatography concluded that the presence of three or more traditional criteria was the best threshold for EUS diagnosis of chronic pancreatitis.53–5564 These studies used pancreatography as a “gold standard”; however, the validity of pancreatography for diagnosis of early chronic pancreatitis is poorly validated: The commonly used Cambridge criteria for pancreatography interpretation are based on expert opinion, and autopsy studies have shown abnormalities on pancreatography in most people without a clinical history of pancreatic disease.62,65
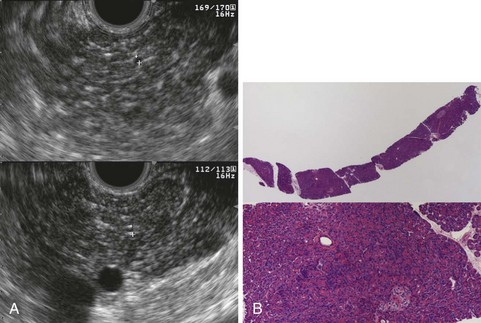
Histologic comparisons have reached conflicting conclusions, with a retrospective study of surgical resection specimens concluding that the best threshold for diagnosis was the presence of three EUS criteria66 and a prospective study of EUS-guided Tru-Cut needle biopsy concluding that histologic abnormalities were uncommon in individuals with three or four traditional EUS criteria (Fig. 41.10).67 This discrepancy likely has several causes, including variations in EUS criteria, tissue sampling error, and differences in patient populations; the first study included patients with chronic pancreatitis of sufficient magnitude to require surgical resection, whereas the second study enrolled patients with chronic abdominal pain and a paucity of other objective findings. These studies are limited by the lack of consensus criteria for histologic diagnosis of chronic pancreatitis. There is histologic overlap between chronic pancreatitis and age-related pancreatic atrophy and fibrosis,62 yet the presence of mild fibrosis was considered sufficient for histologic diagnosis of chronic pancreatitis in the first study.66
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree