Endorectal Advancement Flap
Maher A. Abbas
Matthew J. Sherman
Anal fistula is one of the commonest benign anal disorders, it affects both genders and various age groups. Most anal fistulas are cryptoglandular in origin although other etiologies include Crohn’s disease, obstetrical trauma, radiation therapy, and cancer. Surgical intervention is required to resolve most chronic fistulas. The goal of operation is threefold: eradicate the fistula, preserve continence, and minimize the risk of recurrence. Several options are available to treat anal fistula: fistulotomy; fistulectomy; fistulotomy with sphincteroplasty, seton, injectables such fibrin glue, fistula plugs, and anal flaps. The choice of operation depends on several factors including the anatomy of the fistula and its relationship to the anal sphincter muscles, the etiology of the fistula, the baseline continence level, prior anal operations, and patient’s body habitus. Although most fistulas can be easily eradicated, some can be quite challenging to treat and can carry higher failure and complication rate.
For most low-lying anal fistulas or fistulas involving minimal anal sphincter muscle, fistulotomy can be used with good clinical and functional results. However, for more complex fistulas, especially when there is concern about fecal continence, the endorectal advancement flap is a good option. Closure of the internal fistulous tract by an internal flap can successfully heal most fistulas while at the same time minimizing the risk of anal incontinence. This technique was first described as the sliding flap in 1902 by Noble (1). In his original description, he used a full-thickness rectal flap. In 1948, Laird (2) modified the procedure by advocating the use of a partial-thickness flap consisting of mucosa, submucosa, and a portion of the internal circular muscle. Candidates for the endorectal advancement flap include patients with complex anal fistulas such as high transsphincteric and suprasphincteric fistula, Crohn’s disease–associated fistulas, rectovaginal fistulas, rectouretheral fistulas, fistulas with secondary tracts, and recurrent fistulas (3).
Contraindications to the endorectal advancement flap include acute anal abscess, a strictured anus, scarred rectal wall, and active inflammation in the anorectum from conditions such as Crohn’s disease. In the setting of Crohn’s disease, it is important to determine whether the fistula is a result of active perianal Crohn’s disease or an incidental
finding in a patient with more proximal intestinal disease. In the setting of Crohn’s disease, a delay in wound healing may be noted in up to 80% of patients despite a normal-appearing rectum (4). Not all Crohn’s disease–related anal fistulas require surgical intervention. In one study, spontaneous healing of acute fistulas occurred in 38% of patients without any surgical interventions (5). Fistula closure rates following medical therapy have been reported in 34–50% of patients taking oral metronidazole (6), in 33% of those patients on 6-MP or azathioprine (7), and in as many as 62% of patients who received infliximab (8). In the presence of active proctitis, the success rate of endorectal flap is low with one study reporting closure of fistula in only 20% of patients (9). Under such circumstances, it is best to drain the fistula with a noncutting seton and treat the patient medically. An endorectal advancement flap can be undertaken if and when there is disease remission.
finding in a patient with more proximal intestinal disease. In the setting of Crohn’s disease, a delay in wound healing may be noted in up to 80% of patients despite a normal-appearing rectum (4). Not all Crohn’s disease–related anal fistulas require surgical intervention. In one study, spontaneous healing of acute fistulas occurred in 38% of patients without any surgical interventions (5). Fistula closure rates following medical therapy have been reported in 34–50% of patients taking oral metronidazole (6), in 33% of those patients on 6-MP or azathioprine (7), and in as many as 62% of patients who received infliximab (8). In the presence of active proctitis, the success rate of endorectal flap is low with one study reporting closure of fistula in only 20% of patients (9). Under such circumstances, it is best to drain the fistula with a noncutting seton and treat the patient medically. An endorectal advancement flap can be undertaken if and when there is disease remission.
The evaluation of the patient with anal fistula includes a detailed history of past anal or colorectal operations; past obstetrical history; comorbidities such as diabetes, human immunodeficiency virus (HIV), neurologic or neuromuscular disorders, prior radiation therapy to the pelvis, baseline level of continence, and use of tobacco. Smoking has been associated with a higher failure rate of the endorectal flap and therefore patients who smoke are advised to quit prior to operative intervention (10,11). Physical examination includes visual inspection of the external fistulous opening (location and number of openings if more than one), digital examination to assess sphincter tone, probing of the external opening with the index finger of the examiner inside the anus to evaluate the depth of the fistula and muscle involvement, and anoscopy to visualize the quality of the anorectal mucosa. Occasionally the examination can be limited due to the patient’s discomfort. Under such circumstances an examination under anesthesia is required to ensure patient comfort and cooperation and may reveal an abscess that requires drainage as initial step of treatment. Proper incision and drainage of an underlying abscess yields a higher success rate of the endorectal flap with one study reporting an overall success rate of 73% versus 49% (prior drainage vs. none) (12). The use of noncutting seton prior to definitive endorectal flap improves overall success rate (12). Usually 2–3 months of seton drainage is advised before performing the endorectal flap except for Crohn’s disease where the response of inflammation to the medical therapy is the major determinant of when to proceed with definitive operation.
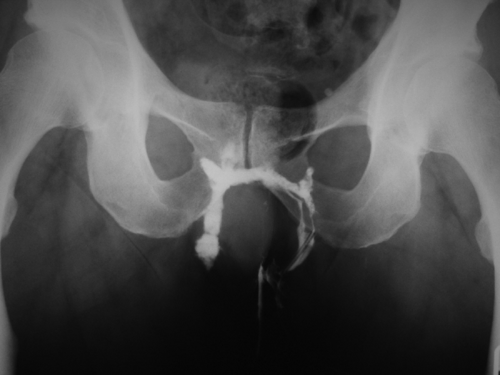
Endoscopic evaluation of the colorectum is indicated in the setting of inflammatory bowel disease or if there is suspicion of it and in patients who meet screening criteria or who warrant diagnostic colonoscopy for history of polyps, cancer, or for symptoms such as bleeding or abdominal pain. Occasionally a high internal fistulous opening can be visualized during the endoscopic retroflexion inside the rectum. Routine imaging of the anorectal area or pelvis to determine the anatomy of a fistula is not warranted. Imaging is used selectively for recurrent or persistent disease, deep tracts, multiple external openings, or when assessing the integrity of the anal sphincter muscles in conditions such as rectovaginal fistula. If an associated sphincter defect is present and a concomitant sphincter repair is performed, success rate of the endorectal advancement flap for rectovaginal fistula is higher. Computed tomography plays little role in the management of anal fistulas but can be helpful in the setting of acute sepsis especially when a deep abscess is suspected. Magnetic resonance imaging and endoanal ultrasound (EUS) are useful diagnostic studies in patients with anal fistulas with similar accuracy. However, EUS is more widely available and more commonly used; EUS is less costly than magnetic resonance imaging and can be performed by the treating surgeon. Fistulous tracts can be confirmed by hydrogen peroxide injection through the external fistulous opening (Fig. 32.1A,B). Fistulogram, while commonly used in the past, is less frequently performed because of the availability of EUS but can be helpful when delineating complex fistulas such as horseshoe (Fig. 32.2).
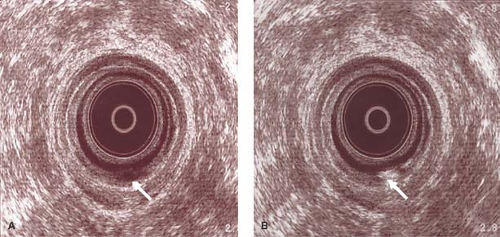 Figure 32.1 A. Ultrasound examination reveals transsphincteric fistula in ano (arrow). B. Hydrogen peroxide confirmed the active tract (arrow). |
Physiologic testing of the anorectum is not routinely performed but can be helpful in a subgroup of patients. Sainio et al. (13) advocate preoperative anorectal manometry in women with previous obstetrical trauma, elderly patients, patients with Crohn’s disease or HIV, or patients with recurrent disease and prior surgery. Preoperative manometric evaluation can elicit sphincter dysfunction already present and may alter the treatment plan.
Bowel Preparation and Antibiotics Prophylaxis
For technical reasons, the rectum needs to be clean at the time of operation. Bowel preparation can be achieved with either oral mechanical bowel preparation the night prior to operation or two sodium phosphates the morning of the operation. Some surgeons prefer a full mechanical bowel preparation citing a better preparation and less infectious complications. No data are available to suggest that one method of rectal cleansing is superior to the other. In our practice, we currently ask the patient to perform two rectal enemas the morning of operation and we have found that approach sufficient for most patients undergoing endorectal advancement flap.
Perioperative use of antibiotics for anorectal surgery has not been well standardized. There are no distinct recommendations from the Surgical Care Improvement
Project. In our practice, we give the patient a single dose of intravenous antibiotics. Appropriate coverage of gram-negative and anaerobic organisms includes single drug regimen such as a second-generation cephalosporin (i.e., cefotetan), ampicillin/sulbactam, or combination of antibiotics such as ciprofloxacin and metronidazole.
Project. In our practice, we give the patient a single dose of intravenous antibiotics. Appropriate coverage of gram-negative and anaerobic organisms includes single drug regimen such as a second-generation cephalosporin (i.e., cefotetan), ampicillin/sulbactam, or combination of antibiotics such as ciprofloxacin and metronidazole.
Anesthesia and Positioning
Choice of anesthesia depends on both surgeon’s preference and patient’s factors. We prefer general anesthesia for most patients as it allows for complete relaxation and cooperation. Alternative to general anesthesia include monitored sedation with local anesthesia, epidural or spinal block.
We perform all of our endorectal flaps in the prone jack-knife position. Once positioned, the buttocks are retracted laterally and secured to the table with tape. We prefer such position as it provides adequate lightening and allows the assistant the proper visualization to provide the exposure and assistance needed during the operation. Some surgeons prefer the lithotomy position for posterior-based fistula.
Surgical Technique
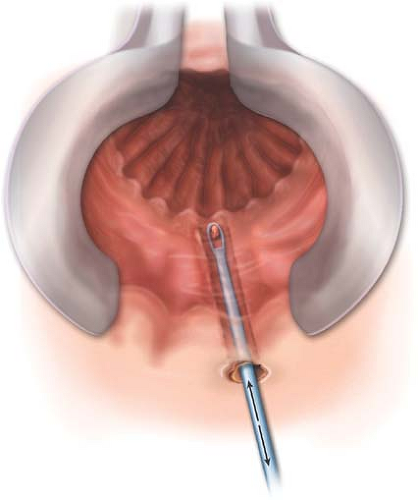
The first step of the operation is to address the fistulous tract. The noncutting seton, if present, is removed. Some authors recommend complete dissection and excision of the fistula tract up to the external anal sphincter (14,15). Curettage of the fistulous tract without excision has been reported by others (16). We prefer to curette rather than excise the tract as to minimize the size of the wound (Fig. 32.3). Curettage of the tract
is performed from the external opening and all the way to the internal opening. Once completed, the tract is flushed with saline to remove any debris.
is performed from the external opening and all the way to the internal opening. Once completed, the tract is flushed with saline to remove any debris.
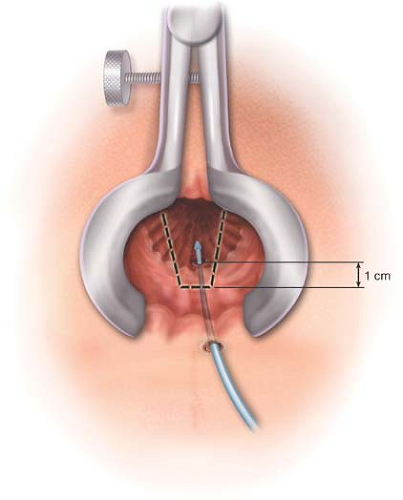
A Pratt Bivalve or Hill-Ferguson anal retractor is used for exposure. Care is taken to not overstretch the anal sphincter complex to minimize injury. We do not use the Parks’ retractor as it can affect resting anal tone (17,18) and may lead to postoperative continence disturbance (14,18). Although we do not use the Lone Star retractor for endorectal flap, it is an alternative to the Pratt Bivalve and Hill-Ferguson retractors and can provide good visualization and decrease the tension on the anal sphincter (18




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree