Fig. 1
Primordial germ cells at their location in yolk-sac as they begin their migration (a). The primordial germ cells migrating through the mesentery to the genital portion of the urogenital ridge bulging into the coelomic cavity (b) modified after Moore et al. [2]
In the 5-week-old embryo (Fig. 2), the two early excretory organ systems (the pronephros and mesonephros) begin to regress. The mesonephros constitutes the lateral portion of the urogenital ridge and consists of mesonephric tubules that interact with glomeruli-like vessels extending from the aorta at one end, and drain into a mesonephric duct at the other end. The regression begins at the cranial aspects and continues toward the caudal end where the duct joins the cloaca (and at what will eventually diverge from the cloaca into the urogenital sinus) . The ureteric bud (also referred to as the metanephric diverticulum) develops as a dorsal bud of the mesonephric duct near its insertion into the cloaca. The metanephros (precursor to the adult kidney) forms from the interaction of the metanephric diverticulum and a region of cells in the intermediate mesoderm known as the metanephric cell mass.
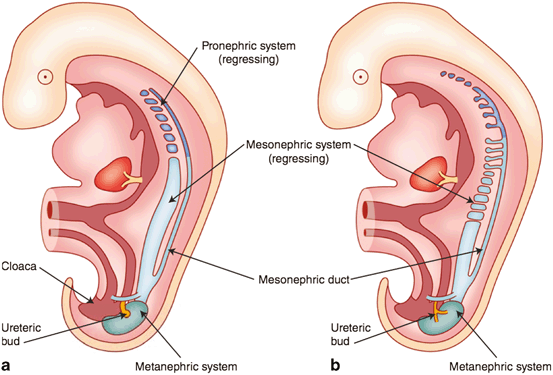
Fig. 2
Development of the early excretory system. Regression of the pronephros (a) and mesonephros (b) with the development of the metanephric system modified after Moore et al. [2]
At 7 weeks (Fig. 3), the embryo is devoid of phenotypic manifestations of sexual differentiation. There are two roughly parallel pairs of genital ducts: in addition to the mesonephric (Wolffian) ducts, there are the paramesonephric (Müllerian) ducts, which are located more laterally. Along the medial portion of the urogenital ridge, cords of coelomic epithelium termed sex cords have invaginated in to the ridge and house the primordial germ cells. This portion of the urogenital ridge becomes the gonadal ridge and the most superficial aspects of the sex cord regress to completely separate the sex cords from the rest of the coelomic epithelium.
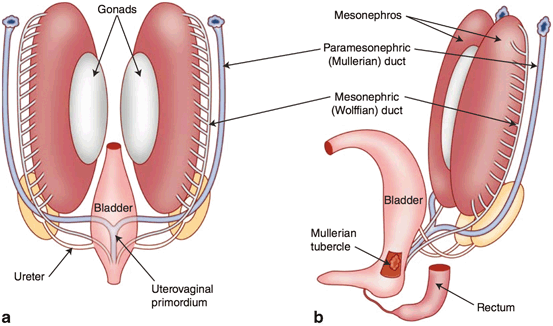
Fig. 3
The reproductive ducts and their relationship to the developing gonads after the incorporation of the distal mesonephric ducts into the urogenital sinus modified after Moore et al. [2]
It is the presence of a Y chromosome gene, called the sex-determining region Y gene (SRY) or testis determining factor (TDF), that results in the development of testes and the initiation of phenotypic sexual differentiation. The testis forms in the gonadal ridge and Sertoli cells differentiate from cells within the epithelial sex cords. By week 8, the developing fetal testis produces at least two hormones. The first has been referred to as Müllerian-inhibiting substance or factor (MIS, MIF) and in some reports, anti-Müllerian hormone (AMH). It is produced by the fetal Sertoli cells and suppresses the development of the paramesonephric duct (which in the absence of this suppression would develop into the upper female reproductive tract organs). The other, testosterone, stimulates the development of the mesonephric ducts into components of the male genital tract. Vestigial remnants of portions of these ducts (Fig. 4a) often can be visualized sonographically.
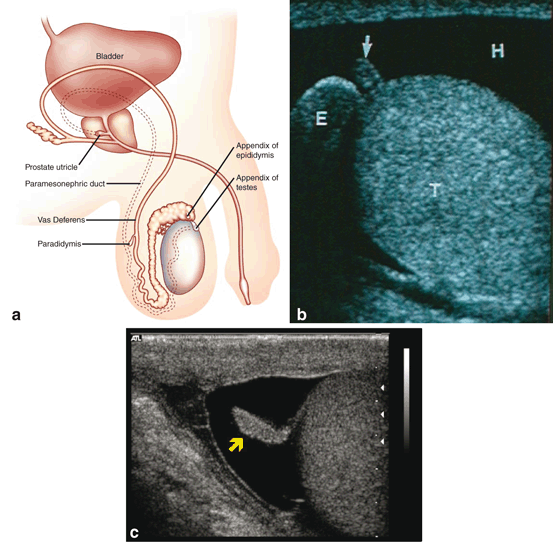
Fig. 4
Locations of the vestigial remnants of the mesonephric and paramesonephric ducts in the male genitalia (a). Ultrasonic appearance of the appendix testis (b) and appendix epididymis (c)
Remnants of the most cranial aspects of the regressed paramesonephric ducts can persist as the appendix of the testis and be demonstrated on ultrasound (Fig. 4b). The most caudal vestigial aspects of the paramesonephric ducts form midline structures and are often found in the prostate, which is derived from the embryonic urogenital sinus . The prostatic utricle is such a structure and can be enlarged and demonstrated on ultrasound. In addition, midline cysts derived from vestiges of the paramesonephric ducts may also be demonstrated. These midline paramesonephric remnants have also been found to obstruct the ejaculatory ducts and result in dilation of the seminal vesicles (anterior–posterior (AP) distance greater than 15 mm).
The deepest aspects of the sex cords converge on what will become the mediastinum testis. The mesenchyme between each of the cords gives rise to the interstitial cells of Leydig and the septa of the testis that radiate out from the mediastinum testis. The sex cords develop into tubules composted of the germ cells and Sertoli cells and the inner ends of these tubes are referred to as the tubuli recti. The gonadal ridge, throughout its development, slowly separates from the mesonephros. The mesonephric tubules adjacent to the testis develop into the ductuli efferentia and meet the tubuli recti at the rete testis. The mesonephric ducts develop into the epididymides, vasa defferentia, and ejaculatory ducts. The seminal vesicles develop as a diverticulum of this tube. Residual mesonephric structures can persist as a vestigial remnant of the mesonephric ducts. Those tubules located cranial to the tubules that become the ductuli efferentia of the testis may protrude off the epididymis as a polypoid vestige called the appendix epididymis (Fig. 4c). In addition, remnant mesonephric tubules can persist proximal to those draining the testicle as well. This remnant is known as the paradidymis (a.k.a. organ of Giraldés), a small collection of convoluted tubules lined by ciliated epithelium, that can be found anywhere along the epididymis or vas deferens.
Torsion of the appendix testis, appendix epididymis, and paradidymis are all in the differential diagnosis of the acute scrotum. The appendix testis, appendix epididymis, and ectasia of the rete tubules can be visualized during the scrotal ultrasound and their characteristic appearance should be kept in mind to avoid confusion of these structures with testicular and extratesticular masses.
Testicular Decent
The process of testicular descent begins prior to 7 or 8 weeks in development. At this time, the gonadal position in the dorsal abdominal wall is similar in both sexes. In males, the fetal testis begins to produce MIS from the Sertoli cells. In addition, androgens and an insulin-like hormone 3 (INSL3) are produced from the Leydig cells. These hormones work in concert to control descent of the testis, which is held by a suspensory ligament at the upper pole, and, at the lower pole by the genitoinguinal ligament, or “gubernaculum.”
During the initial phase of descent, the cranial ligament progresses and the gubernaculum thickens allowing the testis to be held near the inguinal region. At this time the inguinal canal forms as the abdominal wall muscles develop around the end of the gubernaculum.
At about 20–25 weeks an out-pouching of the peritoneal membrane, which is known as the processus vaginalis, travels with the testis toward its final position in the scrotum. The processus vaginalis maintains a connection with both the epididymal tail and the lower pole of the testis. At about 25 weeks, testis and attached processus vaginalis begin to pass through the inguinal canal along with fascial coverings from the abdominal wall (Fig. 5). The processus vaginalis is continuous with the peritoneum and tunica vaginalis of the testis. Between 25 and 30 weeks the testis descends rapidly through the inguinal canal and then more slowly across the pubis into the scrotum. The fascial coverings from the abdominal wall that travel with the testis during its descent become the layers covering the spermatic cord and testis. This process is usually completed by 35 weeks of gestation and it is followed by the obliteration of the proximal portion of the processus vaginalis to close the connection between the scrotum and peritoneum. Closure may occur during prenatal development or in early infancy. The gubernaculum does not become anchored to the scrotum until descent is completed [6−8].
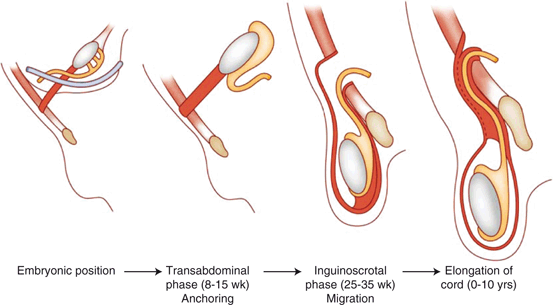
Fig. 5
The phases of testicular descent. At about 25 weeks, testis and attached processus vaginalis begin to pass through the inguinal canal along with fascial coverings from the abdominal wall
The embryology of testicular descent becomes important for the sonographer when evaluating the undescended or nonpalpable testis. A majority of undescended testes are found in the inguinal canal which is accessible to diagnostic ultrasound [9−12]. This makes ultrasound examination of the inguinal region essential when a testis is not found on routine scrotal ultrasound.
Scrotal Contents
The scrotum contains bilateral compartments divided by a septum with multiple fascial layers beneath the skin and dartos fascia. The primary contents of each compartment are a testicle, an epididymis, and a spermatic cord (Fig. 6). The latter contains the ductus deferens, arteries, and veins (the pampiniform plexus). Each testis is covered by a thick, fibrous, connective tissue layer (tunica albuginea). The tunica albuginea is apposed to the visceral tunica vaginalis—one of two, thinner connective tissue layers formed from the distal aspects of the processus vaginalis (Fig. 7). The other is the parietal tunica vaginalis which reflects back off the testicle from the visceral tunica vaginalis to surround the anterior, and lateral portions of the testis creating a cavity that normally contains a small amount of fluid. When this cavity contains more than the physiologic amount of fluid (1–2 mL), a hydrocele is present. Failure of the closure of the processus vaginalis allows peritoneal fluid to accumulate in the scrotum when it is in a dependent location, or when the intra-abdominal pressure rises. This is referred to as a communicating hydrocele. When blood collects in this cavity or in areas outside the parietal vaginalis, it constitutes a hematocele. Cystic dilations of the processus vaginalis maybe found within the spermatic cord on ultrasound examination and are referred to as hydroceles of the cord.
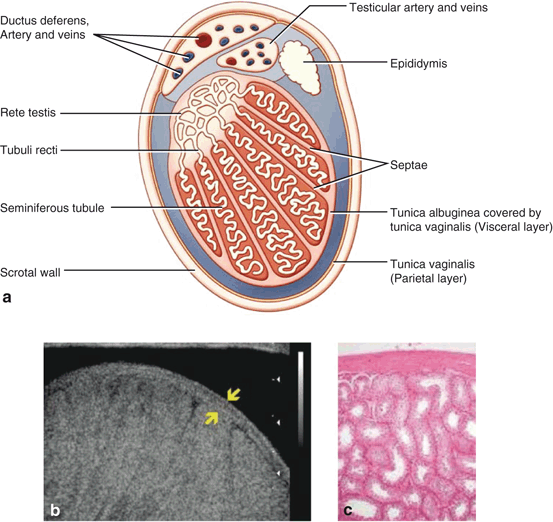
Fig. 6
Cross sectional view of a testis demonstrating the locations of the visceral and parietal layers of the tunica vagnialis (a). Ultrasonogram of the testis demonstrating the tunica albugniea, which is apposed to the visceral portion of the tunica vaginalis (b). Photomicrograph of the spermatic tubules and tunica abuginea (c)
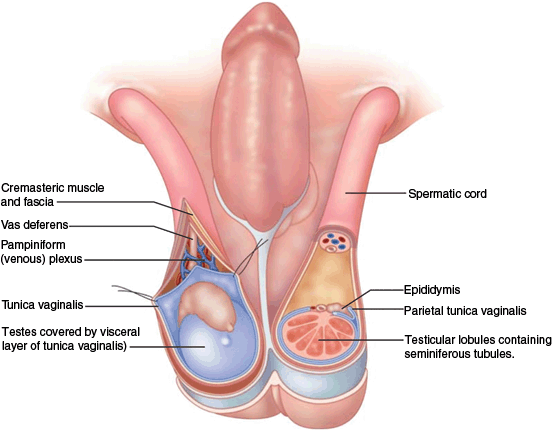
Fig. 7
The contents of the scrotum and spermatic cords. The primary contents of each compartment are a testicle, an epididymis, and a spermatic cord. The latter spermatic cord contains the vas deferens, arteries and veins (the pampiniform plexus)
Development of the Urogenital Sinus
The cloaca is a chamber shared by the allantois (which extends anteriorly from the cloaca into the umbilical cord) and the hindgut . The cloacal membrane makes up the ventral wall of the chamber and is located at the caudal end of the developing hindgut as a bilaminar apposition of ectoderm and the endoderm located on the ventral midline. A septum develops as an ingrowth of folds from the lateral walls and a caudal extension of the intervening mesenchyme from the branch point of the allantois and hindgut, which ultimately divides the cloaca into the anterior/ventral urogenital sinus and the posterior/dorsal developing rectum. While the septum develops, mesodermal mesenchyme also encroaches between the two layers of the cloacal membrane. The septum also divides the membrane into a urogenital membrane and anal membrane. These membranes ultimately rupture to create continuity between the ectoderm and both the urogenital sinus and rectum. The mesenchymal tissues that have encroached develop into the muscles and bones of the lower anterior abdomen and pubis. An incompletely understood defect in this process results in the extrophy–epispadius complex, a spectrum of abnormalities that can include continuities between the luminal surface of the bladder and the lower abdominal skin. If the defect occurs early enough in the process (before the complete division of the cloaca), a cloacal extrophy can occur which also includes a continuity of the intestinal lumen with the skin and bladder lumen. This is often found on prenatal ultrasound with an appearance that mimics the complete absence of a bladder in the fetus. These findings can also be a found with other penile anomalies .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








