CHAPTER 71 Embryology, Anatomy, Histology, and Developmental Anomalies of the Liver
EMBRYOLOGY
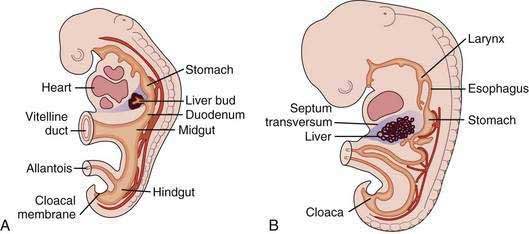
The liver develops at three to four weeks’ gestation as an outgrowing bud of proliferating endodermal cells from the ventral wall of the foregut in response to signals from the adjacent developing heart (Fig. 71-1).1–3 At this stage, the liver bud is separated from the mesenchyme of the septum transversum by basement membrane.2 Shortly thereafter, the basement membrane surrounding the liver bud is lost, and cells delaminate from the bud and invade the septum transversum as cords of hepatoblasts—bipotential cells that will differentiate into hepatocytes and cholangiocytes.1,4 As they invade the septum transversum mesenchyme, the hepatoblasts intermingle with endothelial cells; this interaction is necessary to support hepatic morphogenesis.2
Hepatocytic differentiation involves the development of abundant rough endoplasmic reticulum and the Golgi apparatus needed for synthesis of secreted proteins. The establishment of hepatocyte cell apical-basal polarity begins as early as seven weeks.2,3 Hepatic differentiation is highly dependent on signals from the cardiogenic mesoderm and septum transversum mesenchyme, which produce fibroblast growth factor (FGF) and bone morphogenetic protein (BMP), respectively; these factors induce the expression of hepatic messenger ribonucleic acids (mRNAs).1,4 In the absence of FGF signaling from the cardiac mesoderm, the ventral endoderm develops into pancreas.2
Biliary development begins at six weeks when a subset of hepatoblasts close to the portal mesenchyme strongly express biliary-specific antigens (see Chapter 62).3,5 These biliary precursor cells form a continuous single layered ring around the portal mesenchyme, called the ductal plate. This plate becomes partly bilayered in the next step. A period of remodeling follows in which focal dilations appear between the two cell layers and eventually form lumens. The parts of the ductal plate not involved in the formation of ducts regress by apoptosis, and, around the time of birth, the remaining ducts are incorporated into the portal mesenchyme.5 This process begins in the hilum and extends along a gradient to the periphery of the liver.
The mechanism by which hepatoblasts differentiate into biliary cells is not fully understood. Hepatocyte nuclear factor (HNF)-6 is a transcription factor that regulates the number of cells that enter the biliary differentiation pathway and their localization; Hnf-6-deficient mouse embryos show numerous hepatoblasts that express biliary antigens extending into the liver parenchyma, whereas wild-type mouse embryos have far fewer cells that express biliary antigens and that are restricted to the vicinity of the portal mesenchyme.5 The Notch pathway is also involved in bile duct development; the Jagged1/Notch2 interaction may be critical for induction of biliary differentiation and repression of hepatocytic differentiation.6 Mutations in the gene that codes for the Notch ligand Jagged-1 are associated with Alagille syndrome (see Chapter 62). Longitudinal studies of patients with Alagille syndrome have shown that duct paucity develops progressively after birth, suggesting that the Notch pathway is required to maintain a differentiated phenotype rather than to initiate differentiation and morphogenesis.5 Mutations of several genes, the products of which are involved with ciliary function, lead to disrupted morphogenesis of the bile ducts and cyst formation.7 The extrahepatic bile ducts and gallbladder develop separately and become anastomosed to the intrahepatic bile ducts by an unknown mechanism.
In the initial stages of sinusoidal development, sinusoidal endothelium is continuous and non-fenestrated. This continuous fetal endothelial lining is eventually replaced by a discontinuous fenestrated endothelial lining. In the process, the sinusoidal endothelial cells lose cell markers of continuous endothelial cells, such as platelet-endothelial adhesion molecule-1 and CD34, and acquire markers of adult sinusoidal endothelial cells, such as CD4 and intracellular adhesion molecule-1.8 Thereafter, portal veins develop, followed by centrilobular veins and arteries.
Mesenchymal cells from the septum transversum transform to hepatic stellate cells and fibroblasts.8 Kupffer cells presumably originate from the yolk sac because their presence in fetal liver precedes bone marrow development.1
VASCULAR DEVELOPMENT
The developing liver incorporates the omphalomesenteric (vitelline) and umbilical (placental) veins. The vitelline veins run from the yolk sac to the heart, and as the liver invades them, the midsection of the veins becomes capillarized.9 Portions of the inferior segments of the vitelline veins regress and form a single portal vein, although the right and left vitelline circulations persist as the right and left intrahepatic portal circulations in the adult liver.9 The superior segment of the left vitelline vein regresses and the superior segment of the right vitelline vein becomes the common hepatic vein, which is incorporated into the inferior vena cava.9
The umbilical veins run from the placenta to the heart. During the 6- to 7-mm stage of human development, part of the left umbilical vein becomes the ductus venosus, which shunts placenta-derived arterial blood from the umbilical vein to the inferior vena cava, bypassing the liver; the remainder of the left umbilical vein and the right umbilical vein disappear. After birth, the obliterated prehepatic segment of the left umbilical vein becomes the round ligament of the liver (ligamentum teres hepatis) in the free edge of the falciform ligament, and the ductus venosus becomes the ligamentum venosum.3
ANATOMY
Parietal peritoneum covers the liver except for the bare area, where the liver comes in direct contact with the diaphragm and is suspended by fibrous tissue and the hepatic veins.10 The peritoneal reflections that surround the bare area comprise the superior and inferior coronary ligaments and the right and left triangular ligaments, which attach the liver to the diaphragm.
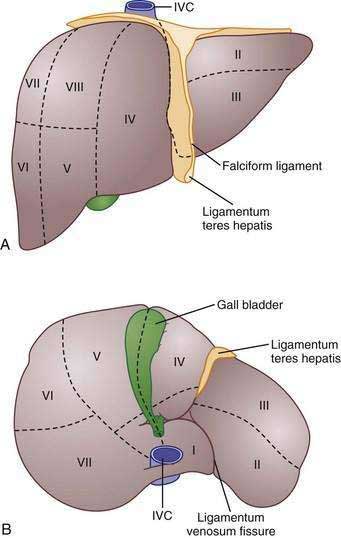
Traditionally, four lobes are distinguished in the liver based on its external appearance: right, left, caudate, and quadrate. On the anterior surface, the falciform ligament divides the liver into the right and left anatomic lobes. On the inferior surface, the quadrate lobe is defined by the gallbladder fossa, porta hepatis, and ligamentum teres hepatis. The caudate lobe is delineated by the inferior vena cava groove, porta hepatis, and ligamentum venosum fissure.11 Although these lobes are convenient and well known, these structures are not true functional lobes.
The true right and left lobes of the liver are of roughly equal size and are divided not by the falciform ligament, but by a plane passing through the bed of the gallbladder and the notch of the inferior vena cava. This plane, which has no external indications, is called the Cantlie line.10,11 Based on arterial blood supply, portal venous blood supply, biliary drainage, and hepatic venous drainage, the liver is divided into right and left functional lobes, each of which is divided into two segments, and these are further subdivided into two subsegments.10 Several systems of subdivision have been proposed, but the most widely used systems are those of Couinaud, which follows the distribution of the portal and hepatic veins,12 and Healey and Schroy, which is based on the distribution of bile ducts.13 In these systems, the subsegments are assigned numbers from 1 to 8, with the caudate lobe being subsegment 1 and the others following in a clockwise pattern (Fig. 71-2).11
The liver receives approximately 70% of its blood supply and 40% of its oxygen from the portal vein and 30% of its blood supply and 60% of its oxygen from the hepatic artery.14 The portal vein is formed from the confluence of the superior mesenteric vein and the splenic vein. At the hilum, the portal vein divides into right and left branches, upon which the right and left lobes of the liver are based.15 The hepatic artery commonly arises from the celiac trunk, although occasionally it arises from the superior mesenteric artery.15 A common variant is a left hepatic artery that branches from the left gastric artery and a right hepatic artery branch that arises from the superior mesenteric artery.15 Within the hilum, the hepatic artery lies anterior to the portal vein and to the left of the bile duct. In the liver, the arteries, portal veins, and bile ducts are surrounded by a fibrous sheath, the Glissonian sheath, whereas the hepatic veins lack this structure.10 Three major hepatic veins drain into the inferior vena cava, although in 60% to 85% of persons, the left and middle veins unite to enter the inferior vena cava as a single vein.10,15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree