Fig. 18.1
Blind Men and the Elephant by John Godfrey Saxe (1816–1887). The story of the blind men and an elephant has been used to illustrate a range of truths and fallacies. At various times it has provided insight into the relativism or inexpressible nature of truth, the behavior of experts in fields where there is a deficit of information. (Reproduced with permission from Himmelfarb J, et al. (2002) The elephant in Uremia, Nature 62:5)
The conditions that are responsible for neuropathic pain are mechanical trauma, ischemia, and degeneration or inflammation of the peripheral or central pain pathway.
In most of cases neuropathic pain is the cause of a CPPS: it is caused as believed in the past by entrapment or more recently by neurogenic inflammation of the pudendal nerve and alteration of pain receptors and central pain pathways by surgery, radiotherapy, or inflammatory diseases [5].
The diagnosis of CPPS is made by exclusion: after ruling out endometriosis, chronic pelvic inflammatory disorders, uterine or ovarian pathology in women, prostatitis in men, urological pathology, interstitial cystitis, or irritable bowel syndrome, remaining patients are diagnosed with a CPPS. Multiple factors are assumed to be involved in the pathogenesis of CPPS, such as chemical irritants, endocrine factors and pelvic floor muscle irregularities, as well as immunological and neurological aspects. The exact pathogenesis of CPPS remains unknown: however, in these patients, pelvic floor hyperactivity and pelvic congestion are common phenomena [6]. CPPS is often related to the dysfunction of the pelvic floor, with associated symptoms such as voiding dysfunction, urinary retention, constipation, and dyspareunia. The pain cycle theory explains why pelvic floor spasms and pelvic pain are linked physiopathologically [7, 8].
No diagnostic tests for peripheral neuropathy are available today for patients with CPPS: somatosensory-evoked potentials of the pudendal nerve and EMG (pudendo-anal reflex) have not been validated at today for this purpose.
Neuropathic pain and CPPS are currently treated with spinal cord and peripheral nerve stimulation by pain clinicians. Several neurophysiological mechanisms of action have been proposed [9]: these include simple blocking of pain transmission by a direct effect in the spinothalamic tracts, activation of descending inhibitory pathways, effect on central sympathetic systems, segmental inhibition through coarse fiber activation and brain stem loops, inhibition by increasing GABA levels in the dorsal horn, and activation of a thalamocortical mechanism masking the nociceptive input.
18.2 Transcutaneous Electrical Nerve Stimulation (TENS)
TENS is a noninvasive therapy used to stimulate peripheral nerve fibers using surface electrodes on the skin. It is a non-pharmacological treatment option in the management of chronic pain. TENS was proposed in the treatment of pelvic pain with simple operation for the patient which is to put self-adhesive electrodes on the skin and then connecting the electrodes to an external stimulating device and to stimulate 20–30 min/day. The electric frequency rate varies from 1 to 100 Hz, depending on the more or less painful response with optimal values around 50 Hz. The position of the electrodes is the most significant variable depending on the painful zone: they can be located on the abdominal wall as well as at the suprapubic level or at a perineal level but also in the inguinal zone. The principle of action is based on the “gate control” theory with a stimulation and recruitment of large myelinated afferent nerve fibers having a fast conduction, inhibiting pain at the spinal level. Sikiru et al. [10] conducted a randomized study versus placebo to evaluate the role of TENS in chronic pelvic pain in men suffering from abacterial prostatitis. TENS stimulation was performed five times a week for 4 weeks for 20 min. The conclusion was that there was a significant improvement in pain in the TENS group (p < 0.005). Fall and Linsdtrom [11] reported their experience on 60 patients testing the efficacy of TENS in the management of “interstitial cystitis.” The stimulation was applied 30 min to two hours twice daily with a follow-up of 9 months to 17 years. They observed a better effectiveness of TENS on pain than in voiding frequency. Similarly, the effectiveness of TENS was 81 % for non-ulcerative interstitial cystitis (27 % remission) against 54 % in case of ulcerative lesion (with 15 % remission).
Schiotz et al. [12] treated 21 patients with “dysmenorrhea” with TENS. Patients were evaluated on the basis of VAS score and analgesic consumption. They observed a significant decrease in VAS score from 6.7 to 5.18 (p = 0.0009) and consumption of analgesics (p = 0.03) with no side effects. Kaplan et al. [13] also showed a decrease in dysmenorrhea pain in 61 patients, with a marked decrease in 30 % and moderate in 60 %. A review of the Cochrane Database [14] was performed in 2002 to assess the effectiveness of TENS and acupuncture in the treatment of dysmenorrhea. The finding was that the high-frequency TENS was effective but that the number of studies were small in size. However, the evidence to conclude about the effectiveness of low-frequency TENS or acupuncture was insufficient.
18.3 Percutaneous Tibial Nerve Stimulation (PTNS)
Nerve stimulation may be performed percutaneously, directly stimulating the nerve fibers. It requires a medical procedure by puncturing with an acupuncture needle and the nerve is then stimulated with an external battery-operating device with fixed parameters (20 Hz, 210 μs, 0–10 V). This stimulation is usually performed on ambulatory basis and can be repeated at home for chronic stimulation with self-adhesive electrodes (Fig. 18.2). The most frequently used stimulation pattern for pelvic pain is the stimulation of the posterior tibial nerve (former popliteal sciatic internal nerve [SPI]) also called percutaneous tibial nerve stimulation (PTNS). The stimulation needle is positioned on the posterior tibial nerve behind the medial malleolus. McGuire et al. [15] were the first to describe this kind of stimulation of the nerve to treat detrusor overactivity. This technique has also been proposed for the treatment of fecal incontinence. In a newer way it is used for the treatment of pelvic pain. Van Balken et al. [16] published in 2003 the first series of the posterior tibial nerve stimulation in the management of pelvic pain. They published a prospective multicenter study in 33 patients with chronic pelvic pain. A subjective response was observed in 42 % of cases. Objectively, a reduction in VAS score more than 50 % was observed in 21 % and a decrease in VAS score more than 25 % in 18 % of cases. They concluded for a small effectiveness of this therapy, but it was considered for future controlled studies.
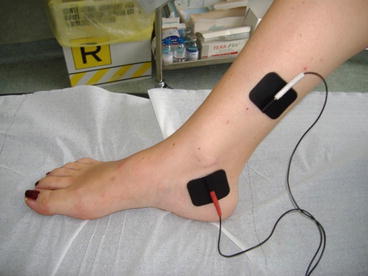

Fig. 18.2
Self-adhesive electrodes positioning for home chronic PTNS
The same authors [17] have published another series trying to evaluate the prognostic factors for the clinical response with the percutaneous nerve stimulation. One hundred thirty-two patients were included in this study: 83 patients with overactive bladder, 16 for chronic non-obstructive retention, and 33 for chronic pain. The overall objective success rate was 32.6 and 51.5 % the subjective rate. The only prognostic objective and subjective factor was poor mental health (depression) assessed by the SF-36, in particular SF-36 Mental Component Summary (MCS).
In another article on a subpopulation of 121 patients in this series [18], they also highlighted a significant improvement in sexual function with PTNS.
Kim et al. [19] also made a prospective study on 15 patients treated with intermittent PTNS for chronic pelvic pain. The conclusions were that after 12 weeks pain improved less than 50 % (assessed by VAS score) in 60 % of cases and improved more than 25 in 30 % of cases with the PTNS.
Similarly Zhao et al. [20] treated 18 patients with painful bladder syndrome/interstitial cystitis with PTNS twice a week for 30 min per session with a total of ten sessions. They did not find significant difference in terms of pain. However, eight patients (44 %) reported improvement in the voided urine volume and found the treatment effective.
More recently Kabay et al. [21] published a controlled study in 89 patients with syndrome chronic pelvic pain/chronic prostatitis treated by PTNS. Patients were randomized to have a nerve stimulation (n = 45) or a treatment with sham (n = 44). An objective response was noted after 12 weeks of stimulation in the group having PTNS with an improvement in pain in 40 % of cases and improvement of symptoms in 66.6 % of cases. No change was observed in the sham group. To date, there is insufficient evidence to determine the role of PTNS in the treatment of chronic pelvic pain. Although initial results seem promising, the populations are small and follow-up durations are relatively short.
18.4 Neuromodulation of Sacral Roots (SNM)
The most accepted neuromodulatory technique is spinal cord stimulation, in which electrical signals are delivered to the spinal cord by electrodes in the epidural space.
Peripheral nerve stimulation is likely to recruit a larger number of nerve fibers for the purpose of activating inhibitory interneurons than spinal cord stimulation, which exerts its effect through layers of dura and cerebrospinal fluid [22]. It also recruits primary afferent delta fibers, which project to the spinothalamic tract and in all probability not to the dorsal column.
There are evidences that dysregulated central nervous system responses may have a major role in the etiology of CPP [23]. These dysregulated responses may maintain the perception of pain in the absence of acute injury. In addition, these changes may enhance perception in such a manner that nonpainful stimuli are perceived as painful and painful stimuli may be perceived as stronger than normal (allodynia and hyperalgesia) [23]. Therefore, it has been suggested that therapies aimed at modulating the nervous system such as centrally acting medications, PTNS, and SNM might be effective.
A possible working mechanism for neuromodulation in the treatment of chronic pain is based on the gate control theory. This theory states that pain perception depends on a pattern of peripheral nervous input. It is supposed that a mechanism at the spinal segment level is present which regulates the interaction between afferent nerve signals and pain sensation [24]. Interneurons of the spinal cord dorsal horn create gating components, and inhibition or facilitation of afferent fibers modulates the input to the spinal transmission neurons. It is also believed that the impulses from the dorsal horn are controlled by a descending system containing fibers from the brainstem, thalamus, and limbic lobes [25]. Neuromodulation is believed to restore the control at the spinal segmental gate as well as at supraspinal sites such as the brainstem and the limbic system nuclei.
Several articles reported on SNM in the treatment of bladder pain syndrome/interstitial cystitis [26–33] with an overall 60 % success rate. Some publications reported about the efficacy of SNM in patients with nonspecific intractable pelvic and/or urogenital pain. Everaert et al. [34] performed a percutaneous nerve evaluation (PNE) in 26 patients after failure of conservative treatment for intractable chronic pelvic pain (including genital, urethral, inguinal, and perineal pain). Patients with interstitial cystitis were excluded from analysis. Significant pain relief was obtained in 16 patients (62 %). Pain relief was significantly better in patients with symptoms of voiding dysfunction than in those with dyspareunia. Relief was also better with decreasing age (p < 0.0001) and better in men than in women (p < 0.05). Of the 11 implanted patients, 8 (73 %) were satisfied with the treatment. Siegel et al. [35] measured the efficacy of SNM in patients with a history of pelvic and/or urogenital pain that persisted for at least 6 months and was refractory to any conventional treatment. Surgical implantation of a definitive neuromodulation device was performed in 10 patients. At a median follow-up of 19 months, 60 % of the patients reported significant improvement in pelvic pain symptoms. Eight patients (80 %) had a decrease in the number of hours of worst pain and nine had an increased number of hours of least pain at long-term follow-up. At baseline the average rate of pain was 9.7 vs. 4.4 at long-term follow-up.
Martellucci et al. [36] analyzed 27 patients affected by pelvic pain tested for sacral nerve modulation. Sixteen patients (59 %) were definitively implanted with a mean follow-up of 37 months. Mean preoperative VAS was 8.1 and decreased to 2.1 ± 1.2 at 6 months follow-up (p < 0.0001), persisting at 1.9 ± 1.3 after 60 months, suggesting that SNM could be effective in the treatment of some patients affected by chronic pelvic pain and the effect persists over time. They also suggested that a positive screening phase and a positive response to gabapentin or pregabalin showed to be predictors of a successful response. Multiple localizations of pelvic pain and pain appeared after stapler surgery seem to be negative factors for the success of the treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






