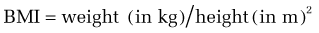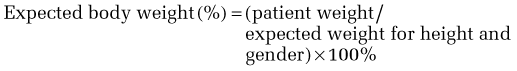CHAPTER 8 Eating Disorders
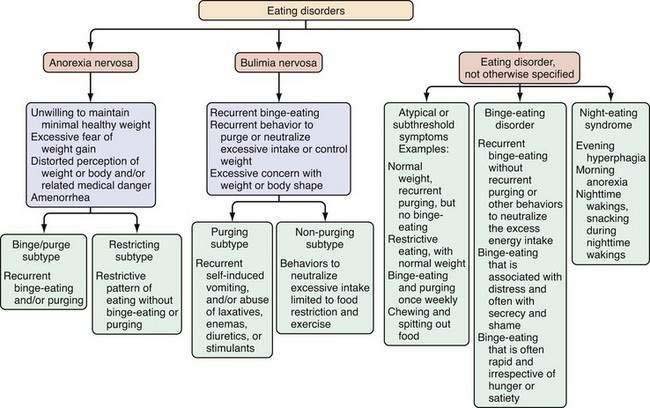
Eating disorders are mental illnesses characterized by disturbances in weight control, body image, and/or dietary patterns. Diagnostic categories include (1) anorexia nervosa (AN); (2) bulimia nervosa (BN); and (3) eating disorder not otherwise specified (EDNOS; Fig. 8-1; Table 8-1). Several variants of EDNOS, such as binge-eating disorder and night eating syndrome, are well-described in the literature. The focus of this chapter is eating disorders in adults; other disturbances in eating that typically have onset in infancy and early childhood, such as pica, rumination syndrome, and feeding disorder of early childhood, are not discussed here. Although eating disorders are classified as mental illnesses, their associated behaviors commonly result in and present with medical sequelae, many of which are gastrointestinal. Because associated chronic undernutrition, overweight, and/or purging behaviors often result in serious medical complications that can be chronic, individuals with eating disorders benefit from the ongoing care of a multidisciplinary treatment team. Indeed, eating disorders (AN and BN) are among the mental disorders with the highest mortality risk.1
Table 8-1 Behaviors Used to Neutralize Excessive Food Intake or to Prevent Weight Gain
| Purging behaviors |
| Non-purging behaviors |
EPIDEMIOLOGY
Eating disorders have been described across diverse global settings, although epidemiologic data are best established for populations in North America and Europe. The incidence rate for AN is approximately eight cases/100,000 population/year, with a point prevalence of AN estimated at 0.3% in young women of the United States and Western European general populations. BN is more common than AN, with an incidence of 12 cases/100,000 population/year in the United States and Western Europe2 and a 12-month prevalence of 0.5% among adult women in the United States. Lifetime prevalence estimates for U.S. women based on the National Comorbidity Study Replication (NCS-R) are 0.9% for AN and 1.5% for BN; U.S. men have a lifetime prevalence of 0.3% for AN and 0.5% for BN.3 The most common presentation of an eating disorder in outpatient settings is EDNOS, although fewer prevalence data are available for this broad and heterogeneous category. One large study from Portugal reported a prevalence of 2.37% for EDNOS in female students in grades 9 to 12.4 Relatively high prevalence rates also are reported for specific symptoms associated with disordered eating. For example, in 2007, 6.4% of school-going female adolescents in the United States reported vomiting or laxative use, and 16.3% reported fasting within the previous month to lose weight.5 Within the diagnostic category of EDNOS, there is great interest in a clinical variant termed binge-eating disorder (BED). The lifetime prevalence of BED in the United States is 3.5% for adult women and 2.0% for adult men. Additional variants of disordered eating (that are not classified as distinct disorders in the Diagnostic and Statistical Manual of Mental Disorders, DSM) include the night eating syndrome (NES) and nocturnal sleep-related eating disorder (NSRED). The prevalence of NES in young adult women has been reported as 1.6%6 and of NSRED as 0.5%.7
Eating disorders occur across ethnically and socioeconomically diverse populations, but each of the eating disorders is more common in women than in men. Males account for less than 10% of individuals with AN, 10% of those with BN, 34% of those with NES, and 40% of those with BED.8,9
CAUSATIVE FACTORS
Although incompletely understood, the cause of eating disorders is almost certainly multifactorial, with psychodevelopmental,10 sociocultural,11 and genetic12 contributions to risk. For example, exposure to risk factors for dieting appears to elevate risk for BN,13 just as childhood exposure to negative comments about weight and shape elevate risk for BED.14 Body dissatisfaction in a social context in which thinness,15,16 self-efficacy, and control are valued may be an important means whereby dieting is initiated and disordered eating attitudes and behaviors ensue. Dietary restraint may precipitate a cycle of hunger, binge eating, and purging.17 Among numerous risk correlates, childhood gastrointestinal (GI) complaints have been found associated with earlier age of onset and greater severity of BN in a retrospective study,18 and picky eating and digestive problems were found prospectively associated with AN in adolescence.10
SATIETY
Serotonin has long been a focus of attention for its possible role in disrupted satiety. There is substantial evidence that altered 5-hydroxytryptamine (5-HT; serotonin) functioning contributes to dysregulated appetite, mood, and impulse control in eating disorders and persists after recovery from AN and BN, possibly reflecting premorbid vulnerability.19,20 There also is evidence that cholecystokinin (CCK) levels are altered in eating disorder populations. Findings for AN are inconsistent: There is some evidence that young women with AN have high levels of pre- and postprandial CCK, which may impede treatment progress by contributing to postmeal nausea and vomiting,21,22 whereas other reports have shown decreased CCK compared with that of controls.23 In those with BN, there is consistent evidence for an impaired satiety response, characterized by a blunted postprandial CCK response and satiety, as well as delayed gastric emptying.24,25 In contrast, individuals with BED and obesity do not differ in postmeal CCK responses from those with obesity but no BED.26 The relationships between CCK, binge eating, and BMI need further clarification. Lastly, protein tyrosine (PYY) functioning also appears to be dysregulated in BN and AN, but not in BED. Young women with AN have higher levels of PYY, the intestinally derived anorexigen that elicits satiety, compared with controls, perhaps contributing to reduced food intake.27 In individuals with BN, expected elevations in PYY after meals are blunted,28,29 possibly playing a role in impaired satiety. A recent report found no differences between BED and non-BED groups in fasting levels and postmeal changes in PYY.30
APPETITE
The orexigenic peptide ghrelin is of interest for its role in eating disorders because it influences secretion of growth hormone (GH), stimulates appetite and intake, induces adiposity, and is implicated in signaling to the hypothalamic nuclei involved in energy homeostasis. There are two consistent findings in the literature examining ghrelin and AN: (1) circulating ghrelin levels are elevated, likely a consequence of prolonged starvation31,32; and (2) GH and appetite responses to ghrelin are blunted, suggesting altered ghrelin sensitivity.33,34 In BN, plasma levels of ghrelin are normal or elevated28,29; of most interest is the postprandial blunted response (i.e., reduced suppression of ghrelin35). Investigations of ghrelin functioning in individuals with BED have reported lower circulating levels of pre- and postmeal ghrelin, possibly reflecting down-regulation in response to chronic overeating and smaller decreases in ghrelin after eating.30
ENERGY STORAGE
Leptin and adiponectin are hormonal signals associated with longer term regulation of body fat stores. Leptin is also directly implicated in satiety through its binding to the ventral medial nucleus of the hypothalamus, an area termed the satiety center. Leptin and adiponectin are both altered in patients with eating disorders. A number of studies have found evidence for hyperadiponectinemia and hypoleptinemia in populations of underweight AN with reversal following restoration of weight36,37; increased adiponectin levels may act protectively to support energy homeostasis during food deprivation. Individuals with BN also exhibit decreased plasma levels of leptin, which are inversely correlated with length of illness and severity of symptoms.38 The mechanism of altered leptin functioning in BN is unclear because blunted postmeal leptin levels are not observed in individuals with BED.26
ONSET AND COURSE
AN and BN most commonly have their onset in adolescence,39 and BED usually manifests in the early 20s,40 but eating disorders can occur throughout most of the lifespan and appear to be increasing in frequency in middle-aged and older women.41,42 Diagnostic migration from one eating disorder category to another is common.43
Lifetime comorbidity of AN, BN, and BED with other psychiatric disorders is high at 56.2%, 94.5%, and 63.6%, respectively.3 Mortality associated with AN and BN combined is five times higher than expected and is one of the highest mortality rates among mental disorders.1 Some data support the chronicity of AN, reporting that slightly under half of survivors with AN make a full recovery, with 60% attaining a normal weight and 47% regaining normal eating behavior; 34% improve but only achieve partial recovery, whereas 21% follow a chronic course.44 Other data suggest that recovery rates for AN may be more favorable than previously believed,3 with one large twin cohort study reporting a five-year clinical recovery rate of 66.8%.45 In contrast, after a five-year follow-up of 216 patients with BN and EDNOS, 74% and 78% of patients, respectively, were still in recovery.46 In a six-year longitudinal study of patients with BED, 43% of individuals continued to be symptomatic.47 In summary, despite well-established treatments available for the eating disorders, up to 50% of treated individuals continue to be symptomatic.48 Some prevention strategies developed for eating disorders show promise, including programs that induce cognitive dissonance about the “thin ideal.”49,50
DIAGNOSIS AND EVALUATION
A substantial percentage of individuals with eating disorders in the United States do not receive treatment for this problem.3 Despite clear diagnostic criteria for the eating disorders, clinical detection often is problematic and up to 50% of cases may go unrecognized in clinical settings. Moreover, individuals with eating disorders are often reluctant to disclose their symptoms,51 and those with BN and BED can have a normal physical examination. Although individuals with AN are underweight by definition, this is easily missed in clinical settings. Even when noted on evaluation, the medical seriousness of low weight is frequently unappreciated.52 Finally, when an eating disorder is suspected or confirmed, patients may decline or avoid mental health care. Indeed, a feature of AN can be denial of the medical seriousness of symptoms.8 Given that many individuals with eating disorders initially present in primary care or medical subspecialty settings, recognition of clinical signs and symptoms across diverse health care settings will facilitate appropriate referrals and make diagnostic evaluation and treatment plans more efficient. One study has reported that individuals with BN are more likely to seek help for their GI complaints prior to seeking treatment for their eating disorder.53 Thus, familiarity with the diagnostic features and gastrointestinal complications of eating disorders will help to identify the most appropriate interventions, including the full spectrum of treatment resources available, for a comprehensive treatment plan.
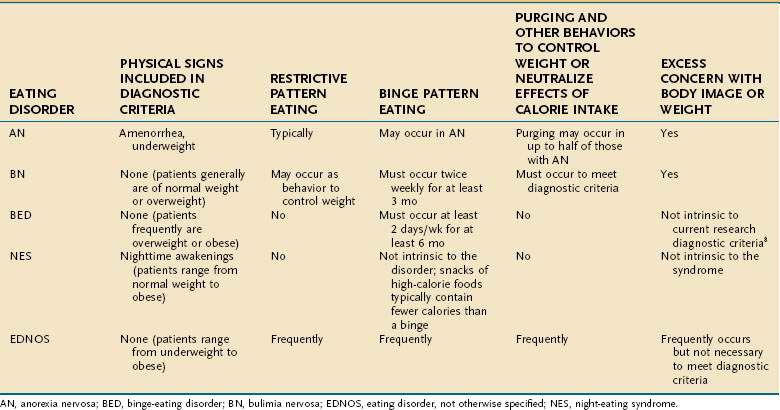
Formal screening for eating disorders can be time-consuming, and although shorter measures are being developed,54 these have many limitations in clinical settings.55 When an eating disorder is suspected, however, a directed clinical interview about restrictive or binge eating and inappropriate compensatory measures to control weight (see Table 8-1) is essential in determining the scope and severity of symptoms that underlie specific GI complaints and pose medical risk.
ANOREXIA NERVOSA
Anorexia nervosa is characterized by an unwillingness or incapacity to maintain a minimally normal weight (commonly described as at least 85% of expected weight or a body mass index [BMI] of 17.5 kg/m2), fear of gaining weight (despite being thin), a disturbance in the way weight is experienced (e.g., a denial of the medical seriousness of being underweight or feeling fat despite emaciation), and amenorrhea (in postmenarcheal females). Individuals with AN typically restrict their food selections and caloric intake, but approximately half of those with AN also routinely binge-eat and/or engage in inappropriate compensatory behaviors, such as induced vomiting or laxative use to prevent weight gain (see Fig. 8-1). AN is subdivided further into restricting type (i.e., those who primarily control their weight through dieting, fasting, or exercising) and binge-eating–purging type (i.e., those who routinely purge calories to control weight and may or may not routinely binge-eat).8 In middle-aged and older women, new-onset AN may present in conjunction with difficulty making life transitions and fear of aging.42 The diagnosis of AN may be delayed when patients present to a GI specialty practice without disclosing their concerns and behaviors relating to weight. Presentation with GI complaints, even if related to real symptoms or disease, can sometimes prove to be a red herring, drawing attention away from and delaying diagnosis of an eating disorder. One study evaluated 20 consecutive patients presenting to a GI practice who were ultimately diagnosed with an eating disorder, and found that patients did not receive a diagnosis of an eating disorder for an average of 13 months after presentation. Notably, all patients stated a desire to gain weight and denied attempts to lose weight via exercise, purging, or dietary restriction.56 Individuals with AN are not always able or willing to frame their difficulty maintaining a healthy weight as intentional; thus, the diagnosis initially may be unsuspected and delayed.
BULIMIA NERVOSA
The clinical hallmark of BN is recurrent binge eating accompanied by inappropriate compensatory behaviors to control weight or to purge calories consumed during a binge. On average, these behaviors must occur twice weekly for at least three months to meet diagnostic criteria.8 Also intrinsic to the diagnosis of BN is the excessive influence of weight and/or shape on self-image.
By definition, binge eating is consumption of an unusually large amount of food during a “discrete period of time” (i.e., not overeating or grazing all day), accompanied by the feeling that the eating cannot be controlled.8 Many patients describe an emotional numbing during the period of eating. For some, this state appears to motivate the bingeing. Most clinicians are familiar with self-induced vomiting as the primary purging behavior, but individuals with BN often use alternative or additional means of preventing weight gain, including abuse of laxatives and/or enemas, diuretics (especially among health care workers), stimulants (including methylphenidate, cocaine, ephedra, and caffeine), underdosing of insulin (for those with diabetes mellitus), fasting or restrictive eating, and excessive exercise (see Table 8-1). Intentional consumption of gluten to promote weight loss in adolescents with celiac disease also has been reported.57
Whereas most compensatory behaviors to prevent weight gain fall within the diagnostic subtype of purging BN, excessive exercise and fasting are behaviors categorized within the subtype of nonpurging BN.8 Because of the absence of the more classic purging behaviors and because of their frequent indistinct nature, this variant of BN often is challenging to identify. As with overeating and dieting, it frequently is difficult to determine the line between culturally normative and pathologic behavior with excessive exercise. Generally, clinical suspicion should be raised when an individual continues to exercise despite an injury or illness, or if he or she is exercising routinely in excess of what a coach is recommending for the team.
It is recommended that clinicians explore the presence of purging behaviors if an eating disorder is suspected. Although it is not certain that a patient will respond candidly, individuals probably are more likely than not eventually to disclose information about symptoms when asked.51 Some patients report feeling relieved when clinicians pose such questions if they previously had not been able to discuss their symptoms. On occasion, however, patients report learning about techniques from clinicians’ questions, so it is advisable to provide a psychoeducational context for the questions (e.g., by conveying serious physical consequences associated with the behavior, such as ipecac use) and to avoid introducing information about a dangerous behavior (e.g., underdosing insulin), depending on the clinical context. Patients also benefit from learning that treatment is available and that their clinicians understand the illness.
Whereas all these purging and other behaviors aimed at neutralizing calorie intake and controlling weight can pose medical risks when chronic, some of them pose more immediate, and potentially lethal, consequences. Patients should be informed of these acute life-threatening risks and steps should be taken to eradicate such behaviors immediately. For example, because of the serious neurotoxicity, cardiotoxicity, and risk of death associated with repeated syrup of ipecac ingestion,58 its ongoing use is a clinical emergency and may require immediate hospitalization. Many patients are unaware of the serious risk associated with syrup of ipecac use. Similarly, ephedra, now banned in the United States, poses risk of stroke or adverse cardiac events, even in young adults.59 Some ephedra-free supplements marketed as weight loss agents also may be proarrhythmic and pose medical risks.60 Although patients find it difficult to abstain from purging behaviors, they may be willing to substitute less immediately harmful behaviors while treatment is initiated.
EATING DISORDER NOT OTHERWISE SPECIFIED
EDNOS covers a broad range of clinical manifestations, including atypical symptoms, symptoms of BED, symptoms consistent with NES, and subthreshold, yet clinically significant, eating disorders. Although EDNOS is a residual category, it nonetheless is the most commonly diagnosed eating disorder in outpatient settings, and therefore there is much interest in refining diagnostic categories of eating disorders.61 EDNOS currently includes eating disorders that do not meet threshold criteria for duration or frequency for AN or BN as well as BED, NES, purging disorder and other atypical variants. Diagnostic classification of eating disorders will be updated in the DSM-V, with an expected publication date of 2012.
BINGE EATING DISORDER
BED is a variant of EDNOS, although there is a substantial literature to support its prevalence and consistent response to specific therapeutic strategies. Like BN, BED is characterized by recurrent binge-eating. To meet provisional criteria for BED, the binge eating episodes must occur two days per week, on average, for at least six months. Unlike BN, however, BED is not associated with recurrent inappropriate compensatory behaviors to prevent weight gain. BED is distinguished from nonpathologic overeating by several possible associated symptoms, including rapid eating, eating irrespective of hunger or satiety, eating alone because of shame, and negative feelings after a binge.8 Apart from overweight or obesity, BED patients frequently present without any specifically associated physical findings. Although in some cases binge eating associated with BED may cause or perpetuate weight gain, many with BED develop symptoms only after they have become overweight. Individuals with BED frequently are distressed enough about their symptoms to seek medical help, although they may present seeking a solution to their weight gain rather than their binge eating. A substantial percentage of patients seeking weight loss treatment will have comorbid BED or NES. Therefore, medical subspecialists are likely to encounter these patients before they have been diagnosed with BED.
NIGHT EATING SYNDROME AND NOCTURNAL SLEEP-RELATED EATING DISORDER
NES is a pathologic eating pattern that may be considered a variant of EDNOS. First described in 1955,62 it, too, is characterized by recurrent bouts of overeating—but not necessarily bingeing—without associated inappropriate compensatory behaviors to prevent weight gain. As such, some individuals may appear to meet criteria for NES and BED, but these are distinct syndromes with relatively little overlap,7,63 and proposed criteria for the syndrome exclude a concomitant diagnosis of BN or BED.64 There is no clear consensus on core criteria for NES, although most investigators propose morning anorexia, evening hyperphagia, and sleep disturbance (operationalized in various ways); some propose the additional criterion of eating in relation to sleep disturbance, such as during a nighttime awakening.65 In one study, NES in obese subjects was associated with an average of 3.6 awakenings/night compared with just 0.3 awakenings/night for matched controls. Subjects with NES ate during 52% of their awakenings, taking in a mean of 1134 kJ/episode, considerably less than the usual intake of a binge associated with BN or BED.64 NES also can occur in nonobese individuals66 but is more common in the obese and may contribute to poor outcome in weight loss treatment programs.64,67 NSRED is also characterized by nighttime snacking, but individuals typically are totally or partially unconscious (e.g., they are in stage 3 or 4 sleep) during the snacking and frequently do not remember it.7
PURGING DISORDER
Emerging evidence raises the possibility of an additional distinctive eating disorder variant characterized by recurrent purging symptoms in the absence of clinically significant binge pattern eating. The proposed name for this diagnostic category is purging disorder. Crossover between this variant and BN appears to be rare, lending support to the hypothesis that this represents a distinctive clinical phenomenon68; data also support comparable severity to BN. Lifetime prevalence of purging disorder has been estimated as 1.1% to 5.3% of young adult women. Course, outcome, and treatment strategies for purging disorder require further research.69
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of the eating disorders includes evaluation and exclusion of medical causes of weight loss, weight gain, anorexia, hyperphagia, vomiting, and other associated symptoms. These considerations are especially germane in cases of atypical or early- or late-onset eating disorders.42 Medical causes of appetite and/or weight loss include hyperthyroidism, diabetes, malignancy, and infectious diseases, among the systemic disorders, and substance abuse, depression, dementia, delirium, and psychosis. Illnesses associated with weight gain include hypothyroidism, Cushing’s disease, and organic brain disease. The differential diagnosis of hyperphagia is broad and includes Prader-Willi syndrome, dementia (including Alzheimer’s disease), and intracranial lesions. Hyperphagia also has been associated with the use of certain medications, particularly many of the psychotropic agents (e.g., lithium, valproate, tricyclic antidepressants, mirtazapine, and conventional and atypical antipsychotic agents), pregnancy,70 and poststarvation refeeding.71
Psychiatric illnesses associated with loss of appetite and weight loss include major depression, anxiety, and substance-use disorders. Moreover, comorbid psychiatric illness is common among those with eating disorders,3 and frequently complicates their diagnosis and treatment. Thus, identification of excessive concern with weight and food intake, unrealistic or inappropriate weight goals, or resistance to attempts to restore normal weight and/or limit excessive exercise can be helpful in distinguishing an eating disorder from another psychiatric illness or in revealing the presence of an underlying comorbid eating disorder. Because individuals with BN and EDNOS can have an unremarkable physical examination on presentation, the diagnosis may remain obscure until the patient discloses his or her symptoms, or until the clinician suspects an eating disorder based on other elements of the clinical history (e.g., weight fluctuations or menstrual irregularities).
Although there potentially is much phenomenologic overlap among the eating disorders and individuals do cross over from one diagnostic category to another (Table 8-2),43 categories are mutually exclusive, according to DSM-IV* diagnostic criteria.8 Even though a transdiagnostic approach to eating disorders classification and treatment has been proposed,72 differing responses to treatment make it desirable to establish a clear diagnosis to optimize care. A weight criterion distinguishes anorexia nervosa from bulimia nervosa in some cases. Individuals who are substantially underweight (e.g., 85% or less of expected body weight) and who otherwise meet criteria for AN most likely should be classified as having AN, even if bingeing, purging, or both are present. Individuals with BED or BN also can have symptom overlap. BN is distinguished by recurrent purging and other behaviors directed at neutralizing excessive calorie intake so as to prevent weight gain, as well as an excessive concern with weight.
NUTRITIONAL EVALUATION
There are several established means for evaluating nutritional status in the office, central to which is measuring weight and height (see Chapter 5). Assessment of the appropriateness of weight for height is one of the key factors intrinsic to determining the urgency of medical and psychiatric care. For patients with AN, it is important not to rely on self-reported weight, given the strong possibility of an inaccurate report. Individuals with AN often go to great effort to conceal their low weights. For example, some patients “water load” prior to a clinical encounter, some attach weights to themselves, and others layer loose and bulky clothing to create the illusion of being of normal weight. Assessment of weight, therefore, should factor in the possibility that a patient may wish to conceal a low weight or weight loss. Some clinicians will find it helpful to have a scale in a private area (i.e., not in a hallway) and a clear and consistent protocol for weighing patients with AN. This might include asking them to void prior to being weighed, to change into a hospital gown, and to remove heavy jewelry. When patients have a history of consuming water prior to an appointment to increase their measured weight, it may be helpful to check a urine specific gravity in order to adjust interpretation of the office weight.
Although BMI may not be an appropriate standard for evaluating a healthy weight status in professional athletes, with relatively high lean muscle mass, and in some ethnic groups (e.g., Polynesians may have a different cut point for obesity73), BMI generally is appropriate for men and women aged 18 years or older. A BMI within the range of 18.5 to 24.9 kg/m2 for men and women is considered normal. A BMI of 17.5 kg/m2 or less is the threshold for meeting the underweight criterion for AN in the ICD-10 (International Classification of Diseases 10).8,74 A BMI in the range of 25 to 29.9 kg/m2 is consistent with overweight, and a BMI higher than 30 kg/m2 reflects obesity.75
Expected weight for height = 100 pounds + 5 pounds per inch above 5 feet ± 10% for women, 106 pounds + 6 pounds per inch above 5 feet ± 10% for men and, if the patient is shorter than 5 feet, then the same number of pounds per inch is subtracted for each inch below 5 feet.76
Although this is a linear equation (compared with the quadratic equation for BMI) and may be less useful at extreme heights, it is straightforward to calculate. Moreover, conceptually, this formula may be easier for patients and families to understand, especially in setting weight goals or limits. A 90% to 110% range of expected body weight is considered within the normal range and is a good place to begin for setting weight gain goals for patients with AN. Within this range, the goal will be refined by clinical history (including the patient’s history of baseline, minimal and maximal weights), whether and when menses return, and medical parameters, such as reversal of bone loss. Patients below 85% of expected body weight likely meet the weight criterion for AN, and those below 75% of expected body weight are seriously nutritionally compromised and generally require inpatient care.77 For patients who are overweight or obese (>110% or >120% expected body weight, respectively), it may not be realistic or desirable to set weight goals within the normal range. Considerations in weight management are discussed subsequently.
Medical Evaluation
Medical evaluation includes a clinical history with special attention to weight fluctuations and any purging or other inappropriate behaviors to neutralize calorie intake to control weight (see Table 8-1). Ascertainment of syrup of ipecac use (as an emetic) and nonadherence to insulin protocols in patients with diabetes mellitus is essential, given the potentially lethal sequelae of these behaviors.78 Symptoms of medical complications of undernutrition, overnutrition, excessive exercise, or purging should be assessed and a menstrual history should be clarified. Physical examination includes a comprehensive assessment of potential complications of nutritional deficiencies, underweight, overweight, excessive exercise, and purging behaviors. If an eating disorder is suspected, physical examination may reveal signs to confirm nutritional compromise (e.g., bradycardia, hypotension, hypothermia, lanugo, breast tissue atrophy, muscle wasting, peripheral neuropathy) or to suggest chronic purging (e.g., Russell’s sign, an excoriation on the dorsum of the hand from chronic scraping against the incisors); hypoactive or hyperactive bowel sounds; an attenuated gag reflex79; dental erosion (perimolysis; Fig. 8-2)80; or parotid hypertrophy (Fig. 8-3).81
Figure 8-2. Dental erosions resulting from chronic vomiting.
(Adapted with permission from the Department of Psychiatry, Massachusetts General Hospital, Boston.)

Figure 8-3. Patient with parotid hypertrophy resulting from chronic vomiting.
(Adapted with permission from the Department of Psychiatry, Massachusetts General Hospital, Boston.)
Medical complications of behaviors associated with AN, BN, BED, and EDNOS are potentially serious and are too numerous to review in detail here; selected complications are listed in Table 8-3. Complications that are common and/or associated with serious morbidity should be actively sought on physical examination and laboratory studies, so that appropriate interventions can be initiated. Examples of such important and common findings include abnormal vital signs (e.g., hypotension, orthostatic hypotension, bradycardia, hypothermia), low weight or overweight, osteopenia or osteoporosis,82 and dental pathology (e.g., perimolysis [erosion of the tooth enamel], caries, or both).81,83,84 Cardiac complications can be lethal and include prolonged QT interval, QT dispersion, ventricular arrhythmias, and cardiac syncope.85,86 Neurologic findings in AN include cortical atrophy and increased cerebral ventricular size.87 Endocrinologic abnormalities include menstrual abnormalities, low serum estradiol levels, low serum testosterone levels, hypercortisolism, and euthyroid sick syndrome, with resultant hypotension and cold intolerance.88 Reported complications of eating disorders during pregnancy include miscarriage, inadequate weight gain of the mother, intrauterine growth retardation, premature delivery, infants of low birth weight and low Apgar scores, and perinatal death.89–92
Table 8-3 Selected Clinical Features and Complications of Behaviors in Patients with Eating Disorders
| SYSTEM AFFECTED | Clinical Feature or Complication | |
|---|---|---|
| ASSOCIATED WITH WEIGHT LOSS AND FOOD RESTRICTION OR BINGE-EATING IN ANOREXIA NERVOSA | SSOCIATED WITH PURGING OR REFEEDING BEHAVIORS IN ANOREXIA NERVOSA, BULIMIA NERVOSA, OR EDNOS | |
| Cardiovascular | ||
| Dermatologic | Russell’s sign (knuckle lesions from repeated scraping against the incisors) | |
| Oral, pharyngeal | Cheilosis | |
| Gastrointestinal* | Anorectal dysfunction | Abdominal pain |
| During refeeding: | ||
| Acute gastric dilatation, necrosis, and perforation | Gastroesophageal reflux | |
| Elevated liver enzyme levels | Gastric necrosis and perforation | |
| Hepatomegaly | Hematemesis | |
| Pancreatitis | Pancreatitis | |
| Prolonged intestinal transit time | ||
| Rectal bleeding | ||
| Rectal prolapse | ||
| Endocrine and metabolic | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |